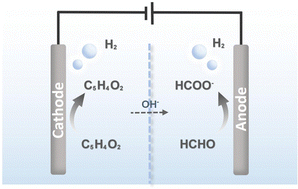
Furfuryl alcohol (FA), formate (FM), and H2 are important chemicals used in various chemical manufacturing industries. One promising approach for the simultaneous production of these chemicals is coupling cathodic furfuryl electrochemical hydrogenation (FEH) with anodic formaldehyde oxidation reaction (FOR) in an electrolyzer using bifunctional electrocatalysts. Here, bifunctional catalysts (CuxAgy/CF) are designed to achieve faradaic efficiency of 95.6 and 100.0% for FA and FM coproduction, respectively. This innovative technology not only achieves more valuable anodic products than oxygen but also simultaneously produces H2 at both anode and cathode at ultra-low voltage. Through density function theory (DFT) calculations and in situ spectroscopy analysis, it is demonstrated that the CuxAgy/CF electrocatalysts optimize the adsorption of intermediates ((C4H3O)CH2O* and H2C(OH)O*) and greatly reduce the energy barriers of rate-determining steps. The Cu3Ag7/CF(+)||Cu7Ag3/CF(−) electrolyzer reaches a current density of 500 mA cm−2 with a cell voltage of only 0.50 V, providing an effective method to simultaneously generate valuable chemicals (FA, FM, and H2).