Deciphering the mechanistic insights of 4-nitrophenol reduction catalyzed by a 1D–2D Bi2S3 nanostructured catalyst†
Abstract
Exploring the reaction mechanism and the role of a catalyst in the conversion of pollutants to value-added products is vital for sustainable development. Herein, a polyvinylpyrrolidone-assisted liquid-phase reflux strategy was utilized to synthesize anisotropic 1D–2D Bi2S3 nanostructures. The as-synthesized nanostructures were used as catalysts in batch experiments for 4-nitrophenol (4-NP) reduction and they exhibited an apparent rate constant (kapp), turnover frequency (TOF), and activation energy (Ea) of 0.441 min−1, 1.543 h−1 and 26.13 kJ mol−1, respectively. Also, the effects of catalyst dosage, NaBH4 amount, 4-NP concentration, solvents, pH, and common ions were evaluated. Isotope labeling and kinetic isotope effects (KIEs) confirm that water is the proton source in 4-NP reduction. Electrochemical studies revealed that the nanostructured 1D–2D Bi2S3 enables the dissociation of BH4− into active absorbed and adsorbed hydrogen (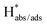 ) species and assists in the catalytic reduction of 4-NP. This study offers a new insight into designing an efficient nanostructured 1D–2D Bi2S3 catalyst for 4-nitrophenol reduction.
) species and assists in the catalytic reduction of 4-NP. This study offers a new insight into designing an efficient nanostructured 1D–2D Bi2S3 catalyst for 4-nitrophenol reduction.



 Please wait while we load your content...
Please wait while we load your content...