Extraction and complexation studies with 2,6-bis(5-(tert-butyl)-1H-pyrazol-3-yl)pyridine in the presence of 2-bromohexanoic acid†
Abstract
To improve the understanding of the extraction chemistry of An(III) and Ln(III) with N-donor ligands 2,6-bis(5-(tert-butyl)-1H-pyrazol-3-yl)pyridine (C4-BPP) in the presence of 2-bromohexanoic acid was investigated. Extraction studies showed an excellent separation factor of SFAm(III)/Eu(III) ≈ 200 and SFAm(III)/Nd(III) ≈ 60 in comparison with the structurally similar ligand 2,6-bis(5-neopentyl-1H-pyrazol-3-yl)pyridine C5-BPP (SFAm(III)/Eu(III) ≈ 100), even though C5-BPP showed significantly higher stability constants. Time-resolved laser fluorescence spectroscopy (TRLFS) studies revealed the formation of the ternary 1 : 1 and 1 : 2 complexes [Eu(C4-BPP)n(2-bromohexanoate)m](3−m)+ (n = 1–2) (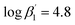 and
and 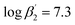 ). [Eu(C4-BPP)2(2-bromohexanoate)m](3−m)+ was the relevant complex species in solvent extraction. In contrast, Cm(III) form stable 1 : 3 complexes. The ability of 2-bromohexanoic acid to replace C4-BPP from the inner coordination sphere of Eu(III) but not from Cm(III) is due to a more favorable complexation of Cm(III) over Eu(III) with C4-BPP. This resulted in a notably more efficient separation of An(III) and Ln(III) in comparison with C5-BPP.
). [Eu(C4-BPP)2(2-bromohexanoate)m](3−m)+ was the relevant complex species in solvent extraction. In contrast, Cm(III) form stable 1 : 3 complexes. The ability of 2-bromohexanoic acid to replace C4-BPP from the inner coordination sphere of Eu(III) but not from Cm(III) is due to a more favorable complexation of Cm(III) over Eu(III) with C4-BPP. This resulted in a notably more efficient separation of An(III) and Ln(III) in comparison with C5-BPP.



 Please wait while we load your content...
Please wait while we load your content...