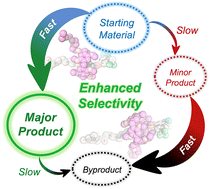
Geometric isomerism in mechanically interlocked systems—which arises when the axle of a mechanically interlocked molecule is oriented, and the macrocyclic component is facially dissymmetric—can provide enhanced functionality for directional transport and polymerization catalysis. We now introduce a kinetically controlled strategy to control geometric isomerism in [2]rotaxanes. Our synthesis provides the major geometric isomer with high selectivity, broadening synthetic access to such interlocked structures. Starting from a readily accessible [2]rotaxane with a symmetrical axle, one of the two stoppers is activated selectively for stopper exchange by the substituents on the ring component. High selectivities are achieved in these reactions, based on coupling the selective formation reactions leading to the major products with inversely selective depletion reactions for the minor products. Specifically, in our reaction system, the desired (major) product forms faster in the first step, while the undesired (minor) product subsequently reacts away faster in the second step. Quantitative 1H NMR data, fit to a detailed kinetic model, demonstrates that this effect (which is conceptually closely related to minor enantiomer recycling and related processes) can significantly improve the intrinsic selectivity of the reactions. Our results serve as proof of principle for how multiple selective reaction steps can work together to enhance the stereoselectivity of synthetic processes forming complex mechanically interlocked molecules.