Dinitrogen reduction chemistry with scandium provides a complex with two side-on (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)2− ligands bound to one metal: (C5Me5)Sc[(µ-η2:η2-N2)Sc(C5Me5)2]2†
N)2− ligands bound to one metal: (C5Me5)Sc[(µ-η2:η2-N2)Sc(C5Me5)2]2†
Abstract
Although there are few reduced dinitrogen complexes of scandium, this metal has revealed a new structural type in reductive dinitrogen chemistry by reduction of bis(pentamethylcyclopentadienyl) scandium halides under N2. Reduction of 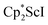 (Cp* = C5Me5) with potassium graphite (KC8) under dinitrogen generates the dark blue paramagnetic complex
(Cp* = C5Me5) with potassium graphite (KC8) under dinitrogen generates the dark blue paramagnetic complex 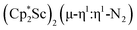 , 1. This end-on bridging (N
, 1. This end-on bridging (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)2− complex is a diradical with a magnetic moment of 2.8µB. Upon further reduction of 1 with KC8, the orange diamagnetic trimetallic complex
N)2− complex is a diradical with a magnetic moment of 2.8µB. Upon further reduction of 1 with KC8, the orange diamagnetic trimetallic complex 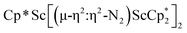 , 2, is obtained. This complex has an unprecedented structure in which two side-on bridging (N
, 2, is obtained. This complex has an unprecedented structure in which two side-on bridging (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)2− ligands are bound to the central (Cp*Sc)2+ moiety. Complex 2 can also be obtained directly from reduction of
N)2− ligands are bound to the central (Cp*Sc)2+ moiety. Complex 2 can also be obtained directly from reduction of 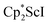 or a mixture of
or a mixture of 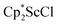 and
and 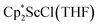 with KC8. The reaction of
with KC8. The reaction of 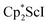 with KC8 in the presence of 18-crown-6 or 2.2.2-cryptand affords 2 along with small amounts of
with KC8 in the presence of 18-crown-6 or 2.2.2-cryptand affords 2 along with small amounts of 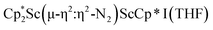 , 3, which is green at room temperature and purple at low temperature and displays a mixture of side-on and end-on bridging isomers in the crystal structure collected at −180 °C. Density functional theory (DFT) calculations are consistent with a triplet ground state for the end-on complex 1 and singlet ground states for the side-on complexes 2 and 3.
, 3, which is green at room temperature and purple at low temperature and displays a mixture of side-on and end-on bridging isomers in the crystal structure collected at −180 °C. Density functional theory (DFT) calculations are consistent with a triplet ground state for the end-on complex 1 and singlet ground states for the side-on complexes 2 and 3.
![Graphical abstract: Dinitrogen reduction chemistry with scandium provides a complex with two side-on (N [[double bond, length as m-dash]] N)2− ligands bound to one metal: (C5Me5)Sc[(µ-η2:η2-N2)Sc(C5Me5)2]2](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D4SC03977G&imageInfo.ImageIdentifier.Year=2024)
- This article is part of the themed collection: 2024 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/h2_char_e001.gif) N)2− ligands bound to one metal: (C5Me5)Sc[(µ-η2:η2-N2)Sc(C5Me5)2]2
N)2− ligands bound to one metal: (C5Me5)Sc[(µ-η2:η2-N2)Sc(C5Me5)2]2