A Bayesian method for selecting data points for thermodynamic modeling of off-stoichiometric metal oxides†‡
Abstract
Thermodynamic characterization of metal oxide reduction/re-oxidation plays a vital role in material identification and optimization of many chemical processes. However, this characterization generally requires significant data collection (spanning several hundred T, pO2, and composition (X) combinations) to appropriately sample phase space and identify key inflection zones that are not known a priori and are missed without sampling on a fine mesh grid of T, pO2, and X combinations. Here we have coupled our previously reported CrossFit compound energy formalism algorithm for reduction/re-oxidation thermodynamic model fitting with Bayesian Inference techniques to build an optimized data selection scheme. Using the BaxSr1−xFeO3−δ system as a proof of concept, we show that our Bayesian data selection technique required less than half (44) data points to achieve the same accuracy as a mesh grid of 100 T, pO2, and X point combinations. Our method has errors of <2 kJ mol−1 in reduction enthalpy 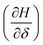 and <3 J (mol−1 K−1) difference in reduction entropy
and <3 J (mol−1 K−1) difference in reduction entropy 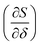 compared to the full data set. Further, 20 instances of a set of 44 randomly selected T, pO2 and X data points only reproduced the ground truth model 5% of the time, demonstrating the power of our approach. Our method offers a human free, physically informed, data collection approach and paves the way for a high-throughput active data selection process for metal oxide reduction/re-oxidation thermodynamics.
compared to the full data set. Further, 20 instances of a set of 44 randomly selected T, pO2 and X data points only reproduced the ground truth model 5% of the time, demonstrating the power of our approach. Our method offers a human free, physically informed, data collection approach and paves the way for a high-throughput active data selection process for metal oxide reduction/re-oxidation thermodynamics.



 Please wait while we load your content...
Please wait while we load your content...