Quantitative rationalization of the unexpectedly moderate water wettability of poly(vinyl alcohol) surfaces: thermodynamic evaluation and prediction of surface hydrogen bonding†
Abstract
In this work, a series of poly(vinyl alcohol) (PVA) films with defined but varied water wettability was prepared by curing as-prepared PVA films at systematically adjusted temperatures. The polar components of surface energy (γs,p) of the resulting PVA films were calculated and correlated with the molecular configurations of their surface OH groups—free OH (OHf), trans-hydrogen bonded OH (OHt), and gauche-hydrogen bonded OH groups (OHg)—with the aid of attenuated total reflectance Fourier transform infrared spectroscopy. By decomposing the γs,p values of the PVA films as a sum of the contributions from OHf, OHt, and OHg groups, the intrinsic γs,p components of 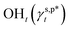 and
and  were calculated to be 8.0 mN m−1 and 9.8 mN m−1, respectively, which were substantially smaller than that of
were calculated to be 8.0 mN m−1 and 9.8 mN m−1, respectively, which were substantially smaller than that of  . This provided a thermodynamic foundation not only to rationalize the unexpectedly moderated surface hydrophilicity of PVA films but also to quantitatively predict the fHB component of hydrogen-bonded OH groups on their surfaces according to their water wettability.
. This provided a thermodynamic foundation not only to rationalize the unexpectedly moderated surface hydrophilicity of PVA films but also to quantitatively predict the fHB component of hydrogen-bonded OH groups on their surfaces according to their water wettability.

- This article is part of the themed collection: Soft Matter 20th Anniversary Collection


 Please wait while we load your content...
Please wait while we load your content...