Mechanisms of oxygen transport resistance of mesoporous carbon-supported catalysts in fuel cells†
Abstract
Understanding the mechanisms of oxygen transport resistance of mesoporous carbon catalysts in proton exchange membrane fuel cells (PEMFCs) is crucial to improve platinum (Pt) utilization. In this work, molecular dynamics (MD) simulations were employed to unravel the origin of local oxygen transport resistance 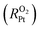 of ionomer–Pt and water–Pt catalysts on mesoporous carbons. It was found that the adsorption resistance (Rads) on Pt surfaces was dominant to
of ionomer–Pt and water–Pt catalysts on mesoporous carbons. It was found that the adsorption resistance (Rads) on Pt surfaces was dominant to 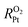 due to the formation of a dense layer. The Rads value of water–Pt catalysts was determined to be 14.32 s m−1, which was much lesser than that of ionomer–Pt catalysts. Besides, we found that Rads would be significantly affected by the presence of carbon supports, ultrathin film effects, or nearby catalysts as the carbon support would present an additional carbon dense layer, the density of which is altered when the film thickness or the distance between two Pt particles is below the threshold value. Furthermore, we calculated the oxygen transport resistance of Pt catalysts in the interior pores of mesoporous carbons, which was in good agreement with theoretical models. We found that the diffusion resistance to the local Pt nanoparticle increases nonlinearly with the depth, and the dense effect of interior Pt will lead to a remarkable increase in
due to the formation of a dense layer. The Rads value of water–Pt catalysts was determined to be 14.32 s m−1, which was much lesser than that of ionomer–Pt catalysts. Besides, we found that Rads would be significantly affected by the presence of carbon supports, ultrathin film effects, or nearby catalysts as the carbon support would present an additional carbon dense layer, the density of which is altered when the film thickness or the distance between two Pt particles is below the threshold value. Furthermore, we calculated the oxygen transport resistance of Pt catalysts in the interior pores of mesoporous carbons, which was in good agreement with theoretical models. We found that the diffusion resistance to the local Pt nanoparticle increases nonlinearly with the depth, and the dense effect of interior Pt will lead to a remarkable increase in 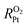 .
.



 Please wait while we load your content...
Please wait while we load your content...