Tyrosine-modified tilapia skin antioxidant peptides and their hydroxyl radical quenching activities†
Abstract
In an antioxidant peptide study, the number and position of active amino acid sites, as well as the peptides’ conformation, are found to be crucial for scavenging hydroxyl radicals (˙OH). Herein, ˙the OH scavenging activity of tilapia pentapeptide (P1, YGDQY) and its analogs including P2 (YYYGDQY), P3 (YYGDQYY) and P4 (YYGPDQYY) was investigated. The results showed that the tyrosine's amount, location and the peptides’ conformation played important roles in determining peptides’ scavenging activity (34.1 ± 0.8%, 45.1 ± 0.9%, 58.6 ± 1.3% and 48.4 ± 0.96% for P1, P2, P3, and P4, respectively). Density functional theory simulation showed that only the tyrosine sites located within the effective diffusion distance 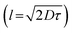 of ˙OH could scavenge the radical. The peptides did not cause cytotoxicity in Caco-2 cells. And the peptide-treated group could increase the activities of glutathione peroxidase (GSH-PX), catalase (CAT) and superoxide dismutase (SOD), and reduced malondialdehyde (MDA) levels. This work may contribute to designing more active antioxidant peptides based on natural peptides’ analogs.
of ˙OH could scavenge the radical. The peptides did not cause cytotoxicity in Caco-2 cells. And the peptide-treated group could increase the activities of glutathione peroxidase (GSH-PX), catalase (CAT) and superoxide dismutase (SOD), and reduced malondialdehyde (MDA) levels. This work may contribute to designing more active antioxidant peptides based on natural peptides’ analogs.



 Please wait while we load your content...
Please wait while we load your content...