Selective formation of rectangular grid coordination polymers with grid dimensions 10 × 15, 10 × 20 and 15 × 20 Å
Kumar
Biradha
* and
Makoto
Fujita
*
Department of Applied Chemistry, Graduate School of Engineering, Nagoya University and CREST, Japan Science and Technology Corporation (JST), Chikusaku, Nagoya, 464-8603, Japan.. E-mail: kbiradha@org.mol.nagoya-u.ac.jp; mfujita@apchem.nagoya-u.ac.jp
First published on 15th December 2000
Abstract
Using four linear bifunctional ligands of different lengths and Ni(NO3)2, three coordination polymers containing big rectangular cavities were shown to form selectively, despite the fact that all the four ligands are known to form square grid coordination polymers when they are reacted independently with M(NO3)2.
The utility of linear bifunctional ligand such as 4,4′-bipyridine 1 has been well explored in the studies of crystal engineering of coordination polymers.1–4 Although there are a considerable number of reports dealing with designing square grid coordination polymers, their rectangular counterparts are not explored to that extent. The synthesis of rectangular grids at will allows the modulation of size and function of the cavity. Only few rectangular grid polymers have been designed either using two charged ligands or using charged and neutral ligands.6–9 However using two neutral ligands to date only one rectangular grid coordination polymer has been reported.10 In this structure the rectangular cavities are too small to enclatharate guest molecules. Recently we have shown that the higher analogues of 1, 1,4-bis(4-pyridyl)benzene 2 and 4,4′-bis(4-pyridyl)biphenyl 3, can also be used in designing open square grid polymers with dimensions of 15 × 15 and 20 × 20 Å.11,12 Herein we show that one can employ these longer ligands to design their rectangular counterparts.
Single crystals of {Ni(1)(2)(NO3)2·6C 6H6}n4 in 62% yield were obtained by layering a MeOH solution (2 ml) of Ni(NO3)2 (6 mg, 0.002 mmol) over a benzene solution (7 ml) of 1 (3.2 mg, 0.002 mmol) and 2 (4.6 mg, 0.002 mmol). Similarly single crystals of {Ni(1)(3)(NO3)2·8C 6H6}n5 were prepared in 76% yield by using 3 instead of 2. The crystal structures of 4 and 5 revealed the selective formation of rectangular grids with the entrapment of benzene molecules.13† In both the complexes the Ni atom has octahedral geometry with four pyridyl groups at the equatorial and two nitrates at the apical positions.
Complex 4 forms 2D-network containing rectangular grids of dimension 11.3 × 15.6 Å and encloses six benzene molecules per metal, two of them are embodied in the rectangular cavities while the other four exist between the layers [Fig. 1(a)].14 Ligands 1 and 2 bridge the metal atoms in the (010) and (001) directions, respectively. The rectangular grid planes sit in (011) plane and pack in the direction of (100). Benzene molecules form a two-dimensional layer [Fig. 1(b)] which is similar to that of naphthalene molecules in the crystal structure of {M(4,4′-bipy)2(NO3)2·3C 10H8}n (M = Co or Ni).15 The network of benzene molecules can be described as one of the semi-regular planar networks, which are described by Wells,16 when benzene molecules are depicted as nodes and aromatic interactions as node connections. The packing of the grids is as follows: the moieties of 1 deposit on each other such that 2,6 and 2′,6′-C–H groups of 1 form C–H⋯O hydrogen bonds (C⋯O 3.126, 3.568 Å; C–H⋯O 151,148°) with the uncoordinated O atoms of the Ni(NO3)2 moiety. As a result this leads to a C–H⋯O hydrogen bonded layer that divides the whole structure into an infinite number of two-dimensional compartments with a width of the longer ligand [Fig. 1(c)]. Each of these compartments accommodates two benzene layers which interpenetrates through the moieties of 2 such that each molecule of 2 interacts with 12 benzene molecules via edge-to-face aromatic interactions.
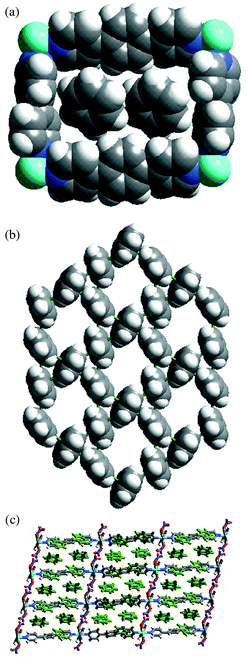 | ||
| Fig. 1 Space filling representations for (a) rectangular grid unit of 4 containing two benzene molecules; (b) layer of benzene molecules exhibited by complex 4; (c) side view (101) of the packing of grids and benzene layers in 4. Note that the C–H⋯O bonded layers and benzene layers (110) are parallel to each other but perpendicular to grid planes (011). | ||
Complex 5 also formed 2D-network containing rectangular grids of dimension 19.9 × 11.3 Å and enclosed eight benzene molecules per metal. Similarly to 4, only two benzene molecules were encapsulated in the cavities while the remaining six exist between the layers (Fig. 2). Ligands 1 and 3 bridge metal atoms in (001) and (100) directions, respectively, and the grid planes pack in the (010) direction. The packing of the grids is similar to that of 4 but now there are three layers of benzene molecules accommodated in each compartment as the width of the compartment is increased from 15.6 to 19.9 Å [Fig. 2(b)]. In this triple layer the outer layers have the same topology as shown in Fig. 1(b) and are linked together by a middle layer, (6,3) net, that is generated by two disordered benzene molecules. In effect each moiety of 3 is surrounded by 18 benzene molecules via edge-to-face aromatic interactions.
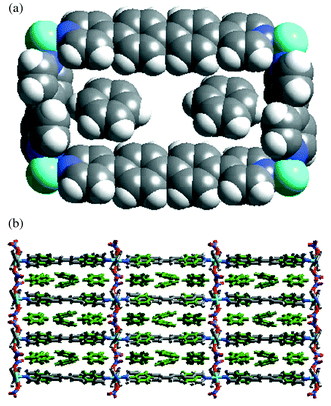 | ||
| Fig. 2 (a) Space filling representation of rectangular grid unit in 5 containing two benzene molecules. (b) Packing of the grids in 5. Note that each compartment accommodates three layers of benzene (disorder of the benzene molecules in the middle layer is not shown). | ||
Our attempts to prepare a 15 × 20 Å grid polymer by using ligands 2 and 3 and Ni(NO3)2/Cu(NO3)2/Co(NO3 )2 were futile as single crystals suitable for X-ray studies were not obtained. Hence we considered 9,10-bis(4-pyridyl)anthracene 6 as a replacement for 2. The layering of an MeOH solution of Ni(NO3)2 over a benzene solution of ligands 3 and 6 resulted in single crystals of the complex {Ni(3)(6)(NO3)(H2O)· 2C6H6·NO3}n 7 (yield 55%).† Single crystal analysis reveals the formation of a coordination polymer containing rectangular grids of dimension 20 × 15.7 Å (Fig. 3). Each grid accommodates four benzene molecules (two are only partly in the cavity), disordered nitrates and H2O. Unlike 4 (8 Å) and 5 (7.8 Å), 7 has an interlayer separation of only 4 Å as no guests exist between the layers. The packing of the grids is different from that of the above structures as the layers here deposit on each other in an offset fashion over the two ligands. It is interesting that the rectangular grids formed selectively from two different ligands although ligands 1–3 and 6 are known to form square grids when they reacted independently with M(NO3)2. Given the big size of these rectangular cavities it is also noteworthy that these grid layers are non-interpenetrated.
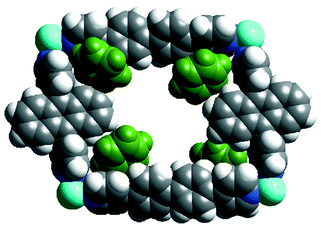 | ||
| Fig. 3 (a) Space filling representation of a rectangular grid unit in 7. Note that this contains four benzene molecules. | ||
Acknowledgements
K. B. thanks the Japan Society for the Promotion of Science (JSPS) for postdoctoral fellowship.Notes and references
- R. W. Gable, B. F. Hoskins and R. Robson, J. Chem. Soc., Chem. Commun., 1990, 1677 RSC.
- M. Fujita, Y. J. Kwon, S. Washizu and K. Ogura, J. Am. Chem. Soc., 1994, 116, 1151 CrossRef CAS.
- S. Subramanian and M. J. Zaworotko, Angew. Chem., Int. Ed. Engl., 1995, 34, 2127 CrossRef CAS.
- S. Kitagawa and M. Kondo, Bull. Chem. Soc. Jpn., 1998, 71, 1739 CAS.
- M. Fujita, Acc. Chem. Res., 1999, 32, 53 CrossRef CAS.
- S. Kawata, S. Kitagawa, M. Konda, I. Furuchi and M. Munakata, Angew. Chem., Int. Ed. Engl., 1994, 33, 1665 CrossRef.
- L. R. MacGillivray, R. H. Groeneman and J. L. Atwood, J. Am. Chem. Soc., 1998, 120, 2677 CrossRef CAS.
- R. H. Groeneman, L. R. MacGillivray and J. L. Atwood, Chem. Commun., 1998, 2735 RSC.
- L.-M. Zheng, X. Fang, K.-H. Lii, H.-H. Song, X.-Q. Xin, H.-K. Fun, K. Chinnakali and I. A. Razak, J. Chem. Soc., Dalton Trans., 1999, 2311 RSC.
- M.-L. Tong, X.-M. Chen, X.-L. Yu and T. C. W. Mak, J. Chem. Soc., Dalton Trans., 1998, 5 RSC.
- K. Biradha and M. Fujita, J. Chem. Soc., Dalton Trans., 2000, 3805 RSC.
- K. Biradha, Y. Hongo and M. Fujita, Angew. Chem., Int. Ed., 2000, 39, 3843 CrossRef CAS.
- The crystals lose the guest molecules at room temperature and become opaque..
- The distances between the metal atoms of the grid were taken as the dimensions of the grid..
- K. Biradha, K. V. Domasevitch, B. Moluton, C. Seward and M. J. Zaworotko, Chem. Commun., 1999, 1327 RSC; K. Biradha, K. V. Domasevitch, C. Hogg, B. Moulton, K. N. Power and M. J. Zaworotko, Cryst. Eng., 1999, 2, 37 CrossRef CAS.
- A. F. Wells, Structural Inorganic Chemistry, Clarendon Press, Oxford, 5th edn., p. 82 [see also p. 613 (MxO3), p. 1006 (β-quartz), p. 1302 (CaCu5)]. Search PubMed.
Footnote |
| † Crystal data for 4: C62H56N6NiO6, monoclinic, space group C2/c, a = 16.123(2), b = 11.341(1), c = 31.106(4) Å, β = 104.190(2)°, U = 5514.0(11) Å3, T = 193 K, Z = 4, Dc = 1.253 g cm−3, λ = 0.7107 Å, 17529 reflections measured, 6477 unique (Rint = 0.0549) which are used in all calculations. R1 = 0.062 and wR2 = 0.141.For 5: C80H72N6NiO6, orthorhombic, space group Pnna, a = 39.824(3), b = 15.5840(9), c = 11.2915(9) Å, U = 7007.7(8) Å3, T = 193 K, Z = 4, Dc = 1.206 g cm−3, λ = 0.7107 Å, 42836 reflections measured, 8301 unique (Rint = 0.0435) which are used in all calculations. R1 = 0.069 and wR2 = 0.173.For 7: C58H44N6NiO7, monoclinic, space group C2/c, a = 15.774(1), b = 19.975(2), c = 16.207(1) Å, β = 106.398(2)°, U = 4898.8(7) Å3, T = 193 K, Z = 4, Dc = 1.350 g cm−3, λ = 0.7107 Å, 15750 reflections measured, 5732 unique (Rint = 0.0560) which are used in all calculations. R1 = 0.069 and wR2 = 0.192. The coordinated H2O and NO3 groups were disordered on two sides of the layer with 0.5 occupancy.CCDC 182/1861. See http://www.rsc.org/suppdata/cc/b0/b007014i/ for crystallographic files in .cif format. |
| This journal is © The Royal Society of Chemistry 2001 |
