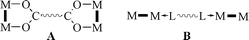Supramolecular structures based on dimetal units: simultaneous utilization of equatorial and axial connections†
F. Albert
Cotton
*a,
Chun
Lin
a and
Carlos A.
Murillo
*a
aLaboratory for Molecular Structure and Bonding and the Department
of Chemistry, Texas A&M University, PO Box 30012, College Station, TX 77842-3012, USA.. E-mail: cotton@tamu.edu; murillo@tamu.edu
bDepartment of Chemistry, University of Costa Rica, Ciudad Universitaria, Costa Rica
First published on 12th December 2000
Abstract
As determined by X-ray crystallography, tetranuclear metal–metal bound molecular loops [Rh2(DAniF)2]2- (O2CCH2CO2)2 (DAniF = N,N′-di-p-anisylformamidinate) react with 2-N donor linkers to give a tubular structure {[Rh2(DAniF)2]2(O2CCH 2CO2)2(NC5H4CHCHC 5H4N)2}n 1, and a sheet-like structure with interstitial dichloromethane molecules for [Rh2(DAniF)2]2(O2CCH2 - CO2)2(NCC6H4CN) 2}n 2; when the assembly unit was changed from [Rh2(DAniF)2(O2CCH2CO2 )]2 to the square [Rh2(DAniF)2(O2CCO2)]4 and the linker NCC6F4C6F4CN was used, the compound {[Rh2(DAniF)2]4(O2C CO2)4(NCC6F4C6 F4CN)4}n 3, was formed, in which there are infinite tubes of square cross section having entrained CH2Cl2 molecules.
Oligomers and polymers in which a key organizing element is a coordinated metal atom, for example, a square-coordinated PdII or PtII atom or a tetrahedral zinc atom, are currently the subjects of vigorous study in many laboratories.1 This work is driven by the search for novel materials, distinguished by special magnetic properties,2 micro- and meso-porous structures,3 or catalytic properties.4
Several years ago we began to explore the possibilities of employing strongly bonded dimetal units, such as Mo24+ and Rh24+, that can be suitably complexed to control their reactivity and then linked to form linear,5a,b square,5c,d triangular5c and polyhedral5e structures. The linking process entails the use of di- and tri-carboxylic acids which attach themselves to adjacent pairs of equatorial sites in the M24+ entities, as shown schematically in A. The arrays that can be built up in this way have as great a range of sizes as those previously made2 but differ from most of those in forming initially as neutral species rather than as highly charges ones. However, charge may readily be introduced—in a controlled, stepwise fashion5b,d—by oxidation of the Mo24+ units.
Here we report that by using axial linking, as shown schematically in B, it is possible to take simple oligomers and connect them to form one- and two-dimensional polymers. These, again, are initially neutral but can be oxidized. This is the first time such architectures have been created by using dimetal building blocks. We also show how the nature of the polymeric structure can be controlled by choosing axial linkers of the right length, a principle that will be of importance in all future work.
In the examples reported here we have utilized dirhodium, Rh24+, building blocks shown as II and III in Fig. 1. The square, III, has been reported before5c but the loop, II, is a new unit, not reported before, though it is somewhat similar to loops that have been made with Mo24+ units.6 Dirhodium units were used here because they have a strong affinity for axial ligands, unlike Mo24+ units. As axial linkers we have employed IV, V, and VI, (Fig. 1).
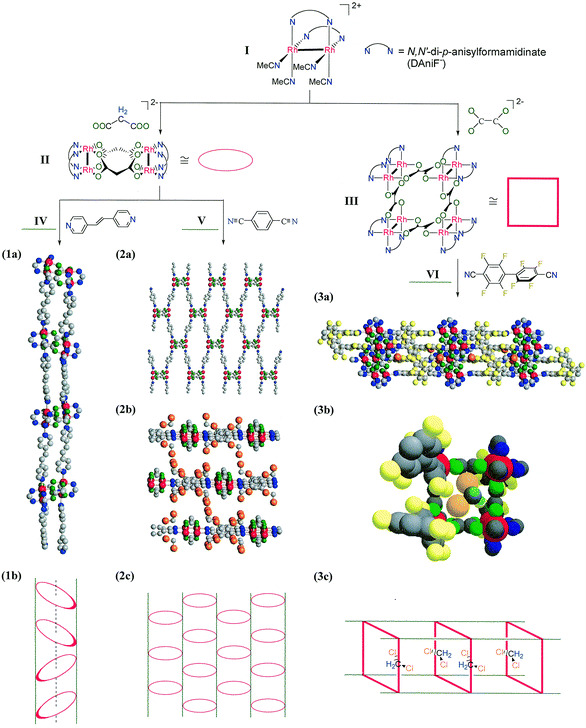 | ||
| Fig. 1 Synthesis of compounds 1·3CH2Cl2·0.5Et2O, 2·4CH2Cl2 and 3·12.36CH2Cl2. Views of the extended structures are given in the middle section as follows: (1a) structure of 1; (2a) structure of 2; (2b) intercalating architecture in 2; (3a) structure of 3 and (3b) a space filling drawing of 3 showing CH2Cl2 molecules inside the square tube. A schematic view of the corresponding structure is shown in the lower section. In the top section, there are axial CH3CN molecules (not shown for clarity) at each Rh atom in I and III. For II, there are two CH3CN molecules distributed on the four Rh atoms. The p-anisyl or DAniF groups have also been omitted for clarity. Color labels: Rh, red; N, blue; O, green; C, gray; F, yellow; Cl, orange; H, turquoise. | ||
With IV, {[Rh2(DAniF)2]2(O2CCH 2CO2)2(NC5H4- CHCHC5H4N)2}n1 is obtained.‡ It has a tubular structure shown in Fig. 1(a). In Fig. 1(b) there is a schematic representation of this structure§ showing how the rings (II) are related alternately by centers of inversion and two-fold axes.
While the formation of such tubes might be considered the ‘obvious’ outcome of linking units of type II by axial bridges, it can only result when the linkers are long enough to keep the bulky p-anisyl groups from clashing with each other. With a shorter linker, V, major clashes would occur and therefore a different structure arises in {[Rh2(DAniF)2]2(O2CCH 2- CO2)2(NCC6H4CN) 2}n2. This sheet-like structure is shown as 2(a), where dichloromethane molecules are omitted, as 2(b) where the sheets are viewed edge-on and the CH2Cl2 molecules are included, and in schematic form as 2(c). The CH2Cl2 molecules were well ordered and refined without difficulty. The sheet belongs to the two-dimensional space group Cmm, the highest symmetry possible in a rectangular sheet structure.
When the assembly unit was changed from II to III and the linker VI was used, the compound {[Rh2(DAniF)2]4(O2C CO2)4(NCC6F4C6 F4CN)4}n, 3 was formed, in which there are infinite tubes of square cross section. A portion of the entire structure is shown as 3(a) while 3(b) shows a portion of one of the units, including one of the entrained CH2Cl2 molecules; 3(c) shows a schematic representation of this structure.
Other studies of these compounds and the syntheses of other assemblies of different topologies containing different dimetal units and axial ligands are in progress.
Acknowledgements
We are grateful to the National Science Foundation for support of this work, and to Johnson Matthey for a generous loan of RhCl3.Notes and references
- See, for example: S. Leininger, B. Olenyuk and P. J. Stang, Chem. Rev., 2000, 100, 853 and references therein; Search PubMed; M. Fujita, Chem. Soc. Rev., 1998, 27, 417 and references therein; Search PubMed; P. Espinet, K. Soulantica, J. P. H. Charmant and A. G. Orpen, Chem. Commun., 2000, 915 Search PubMed; J. A. R. Navarro and B. Lippert, Coord. Chem. Rev., 1999, 185–186, 653 Search PubMed; D. Whang and K. Kim, J. Am. Chem. Soc., 1997, 119, 451 RSC; S. Mann, G. Huttner, L. Zsolnai and K. Heinze, Angew. Chem., Int. Ed. Engl., 1996, 35, 2808 CrossRef CAS; M. Scherer, D. L. Caulder, D. W. Johnson and K. N. Raymond, Angew. Chem., Int. Ed., 1999, 38, 1588 CrossRef CAS; S.-W. Lai, M. C.-W. Chan, S.-M. Peng and C.-M. Che, Angew. Chem., Int. Ed., 1999, 38, 669 CrossRef CAS; C. J. Jones, Chem. Soc. Rev., 1998, 27, 289 CAS; K. K. Klausmeyer, S. R. Wilson and T. B. Rauchfuss, J. Am. Chem. Soc., 1999, 121, 2705 CrossRef CAS; H. Li, M. Eddaoudi, M. O’Keeffe and O. M. Yaghi, Nature, 1999, 402, 276 RSC.
- O. Kahn and C. J. Martinez, Science, 1998, 279, 44 CrossRef CAS; Ø. Hatlevik, W. E. Buschmann, J. Zhang, J. L. Manson and J. S. Miller, Adv. Mater., 1999, 11, 914 CrossRef CAS.
- T. J. Barton, L. M. Bull, W. G. Klemperer, D. A. Lay, B. McEnaney, M. Misono, P. A. Monson, G. Pez, G. W. Sherer, J. C. Vartuli and O. M. Yaghi, Chem. Mater., 1999, 11, 2633 CrossRef CAS.
- M. Fujita, Y. J. Kwon, S. Washizu and K. Ogura, J. Am. Chem. Soc., 1994, 116, 1151 CrossRef CAS.
- F. A. Cotton, C. Lin and C. A. Murillo, J. Chem. Soc., Dalton Trans., 1998, 3151 RSC; F. A. Cotton, J. P. Donahue, C. Lin and C. A. Murillo, Inorg. Chem., in press; Search PubMed; F. A. Cotton, L. M. Daniels, C. Lin and C. A. Murillo, J. Am. Chem. Soc., 1999, 121, 4538 Search PubMed; F. A. Cotton, C. Lin and C. A. Murillo, Inorg. Chem., in press; CrossRef CAS; F. A. Cotton, L. M. Daniels, C. Lin and C. A. Murillo, Chem. Commun., 1999, 841 CrossRef CAS.
- F. A. Cotton, C. Lin and C. A. Murillo, Inorg. Chem., in press; Search PubMed; E. Whelan, M. Deveraux, M. McCann and V. McKee, Chem. Commun., 1997, 427 Search PubMed.
Footnotes |
| † Most of the results reported here were presented in a poster at Contemporary Inorganic Chemistry, II, March 12–15, 2000, College Station, TX, USA. |
| ‡ The general experimental conditions were described in ref. 1. Elemental analyses were satisfactory for all compounds. The following procedure describes the preparation of 1·3CH2Cl2·0.5Et2O. A CH2Cl2 solution (10 mL) of II (82 mg, 0.05 mmol) was carefully layered with a CH2Cl2–Et2O solution (1:1, 20 mL) of trans-1,2-bis(4-pyridyl)ethylene (18 mg, 0.10 mmol). Red crystals formed after several days. A similar method was used for 2·4CH2Cl2 and 3·12.36CH2Cl2. The yields are essentially quantitative. |
§ Crystal data: for
1·3CH2Cl2·0.5Et2O:
C95H95Cl6N12O16.5Rh
4, M = 2293.17, monoclinic, space group
C2/c, a = 56.521(4), b = 19.016(2),
c = 19.564(2) Å, β = 103.053(2)°,
V = 20484(3) Å3, Z = 8,
μ(Mo-Kα) = 0.857 mm−1, T =
213(2) K. The structure, refined on F2, converged for
13458 unique reflections and 613 parameters to give R1 = 0.082 and
wR2 = 0.182 and a goodness-of-fit = 1.031.For 2·4CH2Cl2:
C86H80Cl8N12O16Rh
4, M = 2232.86, triclinic, space group
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 12.708(2), b = 14.378(2),
c = 14.997(3) Å, α = 65.810(3),
β = 72.693(3), γ = 74.766(3)°, V=
2355.0(7) Å3, Z = 1, μ(Mo-Kα) =
0.984 mm−1, T = 213(2) K. The structure, refined
on F2, converged for 6163 unique reflections and 578
parameters to give R1 = 0.051 and wR2 = 0.119 and a
goodness-of-fit = 1.010.For 3·12.36CH2Cl2:
C196.36H144.72Cl24.72F32N
24O32Rh8, M = 5660.01, triclinic,
space group P , a = 12.708(2), b = 14.378(2),
c = 14.997(3) Å, α = 65.810(3),
β = 72.693(3), γ = 74.766(3)°, V=
2355.0(7) Å3, Z = 1, μ(Mo-Kα) =
0.984 mm−1, T = 213(2) K. The structure, refined
on F2, converged for 6163 unique reflections and 578
parameters to give R1 = 0.051 and wR2 = 0.119 and a
goodness-of-fit = 1.010.For 3·12.36CH2Cl2:
C196.36H144.72Cl24.72F32N
24O32Rh8, M = 5660.01, triclinic,
space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 18.235(6), b =
18.430(6), c = 18.665(6) Å, α =
81.839(7)°, β = 81.775(6), γ =
80.742(7)°, V= 6082(3) Å3, Z = 1,
μ(Mo-Kα) = 0.891 mm−1, T =
223(2) K. The structure, refined on F2, converged for
15333 unique reflections and 814 parameters to give R1 = 0.094 and
wR2 = 0.211 and a goodness-of-fit = 1.014. CCDC 182/1852. See
http://www.rsc.org/suppdata/cc/b0/b007347o/ for crystallographic files in
.cif format. , a = 18.235(6), b =
18.430(6), c = 18.665(6) Å, α =
81.839(7)°, β = 81.775(6), γ =
80.742(7)°, V= 6082(3) Å3, Z = 1,
μ(Mo-Kα) = 0.891 mm−1, T =
223(2) K. The structure, refined on F2, converged for
15333 unique reflections and 814 parameters to give R1 = 0.094 and
wR2 = 0.211 and a goodness-of-fit = 1.014. CCDC 182/1852. See
http://www.rsc.org/suppdata/cc/b0/b007347o/ for crystallographic files in
.cif format. |
| This journal is © The Royal Society of Chemistry 2001 |