Unprecedented stabilisation of the Ag22+-ion by two hydrido-iridium(III) complexes
Michael
Gorol
,
Nadia C.
Mösch-Zanetti
,
Herbert W.
Roesky
*,
Mathias
Noltemeyer
and
Hans-Georg
Schmidt
Institut für Anorganische Chemie der Universität Göttingen, Tammannstr. 4, D-37077, Göttingen, Germany. E-mail: hroesky@gwdg.de
First published on 21st November 2002
Abstract
The complex [{(η5-C5Me5)(Ph3P)Ir(μ-H)2}2Ag2(OSO2CF3)2], containing the Ag22+-ion, has been synthesized; crystallographic and spectroscopic data are described.
There is considerable interest in theory and evidence of d10–d10 closed-shell attractions.1 Such metallophilic attractions are found both in organometallic and inorganic compounds.2 Therein, Ag+–Ag+ distances below 288.9 pm as in metallic silver3 are of particular interest. So far, the shortest contact of 267 pm was found in the bis[1,3-diphenyltriazenido-silver(I)].4 Ag+-ions are known to form adducts with transition-metal fragments, such as in [{(η5-C5Me5)(CO)2Ir}2Ag][BF4]5 with Ag–Ir interactions and, more often as in [(Ph3P)3Ir(μ3-H)(μ-H)2Ag2(OSO2CF3)(H2O)](CF3SO3)6 or [(Ph3P)Ag(μ-H)IrH2(PPh3)3](CF3SO3)7 with hydrido-bridged Ag–(μ-H)–Ir linkages. The capability of many transition-metal hydrides to combine with electron-deficient species forming bimetallic complexes of the type M–(μ-H)–M′ is well established.8 However, stabilisation of d10–d10 closed shell units by transition-metal hydrides has as yet not been reported. Herein, we describe the synthesis, crystal structure analysis and spectroscopic characterisation of the first example of a Ag22+-ion coordinated by two hydrido-iridium(III) complexes in [{(η5-C5Me5)(Ph3P)Ir(μ-H)2}2Ag2(OSO2CF3)2] (2).
Addition of 1 equiv. of AgOSO2CF3 to the iridium(III) complex (η5-C5Me5)(Ph3P)Ir(OEt)(H)9 (1) in dichloromethane solution readily affords [{(η5-C5Me5)(Ph3P)Ir(μ-H)2}2Ag2(OSO2CF3)2] (2), which is isolated as a greenish-yellow crystalline solid in 85% yield (Scheme 1).† Formation of 2 occurs by β-elimination of ethoxide forming intermediate (η5-C5Me5)(Ph3P)IrH2 (3) which reacts with AgOSO2CF3. Evidence for the formation of acetaldehyde was given by small resonances in the NMR spectra. Solutions of 2 in dry dichloromethane remain unchanged for several days, but upon contact with moisture decomposition occurs indicated by deposition of a black residue.
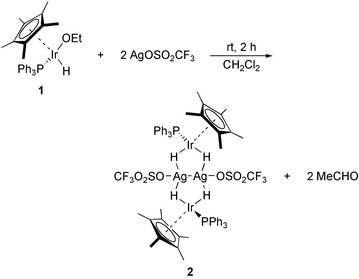 | ||
| Scheme 1 | ||
The infrared spectrum exhibits very weak bands in the typical region of transition-metal hydride absorptions.10 Absorption bands at 2036 and 2021 cm−1 for hydrides bonded terminal to iridium appear together with a broad band at 1917 cm−1 attributable to bridging Ir–(μ-H)–Ag linkages. The 1H NMR spectrum of 2 shows the resonance for the protons of the η5-C5Me5 groups at δ 1.90 (br, d, JH–P 2.3 Hz, 30H) and one for the four hydrides at δ −14.57 (d, JH–P 23.5 Hz, 4H). The comparison with the dihydrido complex (η5-C5Me5)(Ph3P)IrH2 (3) (δ 1.90, br d, JH–P 1.0 Hz, C5Me5; δ −16.5, d, JH–P 31.7 Hz, IrH2)11 shows that the electron densities at the iridium centres are similar and less affected by the coordination to the silver atoms. In contrast, the resonance for the four hydrides in 2 is shifted to lower field in comparison to 3, consistent with a decreased electron density as a result of the interaction with the silver atoms. This is also in good agreement with a stronger acidity of bridging hydrides in comparison to terminal ones at the same transition-metal centre.12 NMR analysis shows the hydrides in 2 to be magnetically equivalent probably due to dynamic processes in solution. Variable temperature 1H NMR spectroscopy was performed on a sample of crystalline 2 in CD2Cl2 between 283 and 188 K (Fig. 1). The doublet for the hydrides in the 1H NMR spectrum at 301 K splits on cooling. At 203 K the resonances at δ −14.79 (JH–P 25.6, JH–Ag 59.6 Hz) and at δ −14.32 (JH–P 24.4, JH–Ag 35.8 Hz) are compatible with an A2MX and an A2A2′MM′XX′ spin system, respectively, together with a broad signal at δ −14.06. At lower temperatures the resonances did not resolve further. The relative intensities are of 0.7, 2.9 and 0.4 versus 30 protons of the η5-C5Me5 groups. The second resonance is consistent with the solid-state structure of 2 showing the coupling to phosphorous and to two equivalent silver nuclei. The first resonance suggests the presence of a phosphorous-containing species with one silver atom such as 2a, whereas the broad resonance is probably due to polymeric material (Scheme 2). Unambiguous determination of the H–Ag couplings occurred by phosphorus-decoupling of the 1H NMR spectrum at 203 K, causing selective collapse of the signals to the expected A2X and A2A2′XX′ spin systems, respectively. The broad signal remains unchanged. Separate 1H–109Ag and 1H–107Ag couplings were not resolved, similar to comparable structural fragments reported elsewhere.13,14 At 301 K the 31P{1H} NMR spectrum shows a singlet at δ 8.8 (δν1/2 9 Hz), which splits into three broad resonances at δ 14.1 (δν1/2 25 Hz), 11.4 (δν1/2 165 Hz) and 9.4 (δν1/2 110 Hz) on cooling. Phosphorous–silver couplings were not observed consistent with previously described examples, such as [AgIr(μ-H)2(bpy)(PPh3)2]2+.13
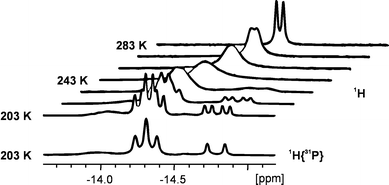 | ||
| Fig. 1 Variable temperature 1H NMR spectra and 1H{31P} NMR spectrum at 203 K of 2 showing the hydride region recorded in CD2Cl2. | ||
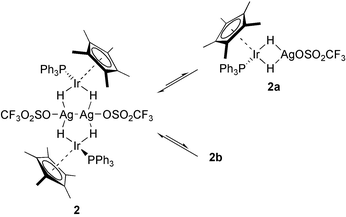 | ||
| Scheme 2 | ||
Single crystals suitable for X-ray diffraction analyses of 2 were obtained by slow diffusion of diethyl ether into a concentrated dichloromethane solution.‡Fig. 2 shows the molecular structure of [{(η5-C5Me5)(Ph3P)Ir(μ-H)2}2Ag2(OSO2CF3)2] (2) along with selected bond lengths and angles. The complex consists of centrosymmetric dimers. The Ir–Ag2–Ir core exhibits a planar rhombic geometry with Ir–Ag–Ir and Ag–Ir–Ag angles of 126.95(2)° and 53.05(2)°, respectively. The most important feature of the structure is the very short silver–silver bond length of 265.53(12) pm, which is comparable to the so far shortest of 266.86(1) pm in [Ag(PhNNNPh)]2.4 The silver ions are additionally involved in weak contacts to anionic oxygen atoms of the CF3O2SO− groups forming an O–Ag–Ag–O chain deviated from linearity by approximately 15°. The Ag–O bond length of 239.2(7) pm is in good agreement with that found in [Ag3(OSO2CF3)3(PPh3)3].15 The iridium–silver distances are 291.07(11) pm and 303.03(9) pm. These values are comparable to previously determined Ag–(μ-H)–Ir linkages of 280.8(4) pm and 276.4(4) pm in [(Ph3P)3Ir(μ3-H)(μ-H)2Ag2(OSO2CF3)(H2O)](CF3SO3).6 A significantly shorter bond length of 265.9(1) pm was determined for a direct Ag–Ir bond interaction in [{(η5-C5Me5)(CO)2Ir}2Ag][BF4],5 which is close to the sum of covalent radii of iridium and silver (261 pm).16 The hydride ligands could not be positioned on the basis of the data obtained by single crystal diffraction methods, due to the absence of rest electron density.
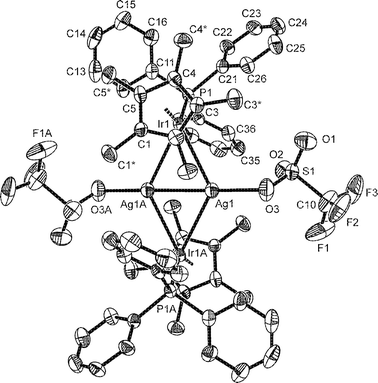 | ||
| Fig. 2 Molecular structure of 2. Selected bond lengths (pm) and angles (°): Ag1–Ag1A 265.53(12), Ir1–Ag1 291.07(11), Ir1–Ag1A 303.03(9), Ag1–O3 239.2(7), Ir1–P1 226.3(2); Ir1–Ag1–Ir1A 126.95(2), Ag1–Ir1–Ag1A 53.05(2), O3–Ag1–Ag1A 165.5(2), P1–Ir1–Ag1 112.72(5), P1–Ir1–Ag1A 107.71(5). ). Symmetry transformations used to generate equivalent atoms A: −x + 1, −y + 2, −z + 1. | ||
The crystal structure of 2 provides the first structural evidence of argentophilic§ attraction in closed shell systems stabilised by transition metal fragments. The formation of the (η5-C5Me5)(Ph3P)IrH2 units in 2 is clearly demonstrated by IR and NMR spectroscopy. However, the nature of the hydride bonding in 2 is not certain. Spectroscopic data suggest bridging Ir–(μ-H)–Ag interactions rather than mixed bridging Ir–(μ-H)–Ag and terminal Ir–H bonds. The stabilisation of the Ag22+-ion would be possible by interaction with these hydrides. Substitution of iridium by other group 9 metal centres stabilising further d10–d10 systems might be helpful for the understanding of this kind of metallophilic attraction. Research in this field is in progress.¶
The Deutsche Forschungsgemeinschaft, the Degussa-Hüls AG, and the Fonds der Chemischen Industrie supported this work.
Notes and references
- P. Pyykkö, N. Runeberg and F. Mendizabal, Chem. Eur. J., 1997, 3, 1451 CrossRef CAS; L. Carlucci, G. Ciani, D. W. v. Gudenberg and D. M. Proserpio, Inorg. Chem., 1997, 36, 3812 CrossRef CAS; M. Jansen, Angew. Chem., 1987, 99, 1136 CAS; M. Jansen, Angew. Chem., Int. Ed. Engl., 1987, 26, 1089 CrossRef; Y. Jiang, S. Alvarez and R. Hoffmann, Inorg. Chem., 1985, 24, 749 CrossRef CAS; P. K. Mehrotra and R. Hoffmann, Inorg. Chem., 1978, 17, 2187 CrossRef CAS; A. Dedieu and R. Hoffmann, J. Am. Chem. Soc., 1978, 100, 2074 CrossRef CAS.
- P. Pyykkö, J. Li and N. Runeberg, Chem. Phys. Lett., 1994, 218, 133 CrossRef.
- L. Pauling, in Die Natur der Chemischen Bindung, Verlag Chemie, Weinheim 1968 Search PubMed.
- J. Beck and J. Strähle, Z. Naturforsch., Teil B, 1986, 41, 4 Search PubMed.
- F. W. B. Einstein, R. H. Jones, X. Zhang and D. Sutton, Can. J. Chem., 1989, 67, 1832 CAS.
- P. Braunstein, T. M. Gomes Carneiro, D. Matt, A. Tiripicchio and M. Tiripicchio Camellini, Angew. Chem., 1986, 98, 721 CAS; P. Braunstein, T. M. Gomes Carneiro, D. Matt, A. Tiripicchio and M. Tiripicchio Camellini, Angew. Chem., Int. Ed. Engl., 1986, 25, 748 CrossRef.
- A. Albinati, C. Anklin, P. Janser, H. Lehner, D. Matt, P. S. Pregosin and L. M. Venanzi, Inorg. Chem., 1989, 28, 1105 CrossRef CAS.
- L. M. Venanzi, Coord. Chem. Rev., 1982, 43, 251 CrossRef CAS; G. G. Hlatky, B. F. G. Johnson, J. Lewis and P. R. Raithby, J. Chem. Soc., Dalton Trans., 1985, 1277 RSC.
- D. S. Glueck, L. J. Newman Winslow and R. G. Bergman, Organometallics, 1991, 10, 1462 CrossRef CAS.
- C. B. Cooper, D. F. Shriver and S. Onaka, Adv. Chem. Ser., 1978, 167, 232 Search PubMed.
- A. H. Janowicz and R. G. Bergman, J. Am. Chem. Soc., 1983, 105, 3929 CrossRef CAS.
- R. G. Pearson and P. C. Ford, Comments Inorg. Chem., 1982, 1, 279 Search PubMed.
- B. D. Alexander, B. J. Johnson, S. M. Johnson, P. D. Boyle, N. C. Kann, A. M. Mueting and L. H. Pignolet, Inorg. Chem., 1987, 26, 3506 CrossRef CAS.
- L. F. Rhodes, J. C. Huffman and K. G. Caulton, J. Am. Chem. Soc., 1984, 106, 6874 CrossRef CAS.
- R. Terroba, M. B. Hursthouse, M. Laguna and A. Mendia, Polyhedron, 1999, 18, 807 CrossRef CAS.
- R. J. Puddephatt and P. K. Monaghan, The periodic table of the elements., 2nd edn., Clarendon Press, Oxford, 1986 Search PubMed.
- H. Schmidbaur, Chem. Soc. Rev., 1995, 24, 391 RSC.
Footnotes |
| † Selected analytical data of 2: Calc. for C58H64Ag2F6Ir2O6S2P2: C, 41.04; H, 3.80; found: C, 41.34; H, 3.97%. MS (EI) m/z: 1024 (100%) [M − 2AgOSO2CF3 − 2C6H5]+, 512 (20%) [M − 2AgOSO2CF3 − 2C6H5]2+. 1H NMR (200.13 MHz, CD2Cl2): δ 7.6–7.2 (m, 15H, P(C6H5)3), 1.90 (br d, 15H, JH–P 2.3 Hz, C5(CH3)5), −14.57 (d, 2H, JH-P 23.4 Hz, IrH2). 13C NMR (125.77 MHz, CD2Cl2): δ 133.56 (d, JC–P 10.6 Hz, P(C6H5)3), 131.30 (d, JC–P 2.5 Hz, P(C6H5)3), 128.97 (d, JC–P 10.7 Hz, P(C6H5)3), 121.01 (q, JC–F 320.2 Hz, SO3CF3), 96.51 (s, C5(CH3)5), 11.03 (d, JC–P 0.5 Hz, C5(CH3)5).19F NMR (188.30 MHz, CD2Cl2, ext. C6F6): δ 84.7. 31P NMR (81.01 MHz, CD2Cl2): δ 8.77 (br s). IR (KBr) 2036, 2021, 1917 (cm−1). |
‡ Crystal data for 2: C58H64Ag2F6Ir2O6S2P2, M = 1697.29, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 1013.0(2) pm, b = 1118.6(3) pm, c = 1447(4) pm, α = 71.179(19)°, β = 72.004(11)°, γ = 89.714(11)°, V = 1.4686(7) nm3, T = 200(2) K, Z = 1, F(000) = 824. Data were collected in the range 3.65 to 25.02° (θ-scan), 5154 independent reflections (Rint = 0.1175), final R1 = 0.0404, with allowance for thermal anisotropy of all non-hydrogen atoms. CCDC reference number 194589. See http://www.rsc.org/suppdata/cc/b2/b209884a/ for crystallographic data in CIF or other electronic format. , a = 1013.0(2) pm, b = 1118.6(3) pm, c = 1447(4) pm, α = 71.179(19)°, β = 72.004(11)°, γ = 89.714(11)°, V = 1.4686(7) nm3, T = 200(2) K, Z = 1, F(000) = 824. Data were collected in the range 3.65 to 25.02° (θ-scan), 5154 independent reflections (Rint = 0.1175), final R1 = 0.0404, with allowance for thermal anisotropy of all non-hydrogen atoms. CCDC reference number 194589. See http://www.rsc.org/suppdata/cc/b2/b209884a/ for crystallographic data in CIF or other electronic format. |
| § In the case of gold, the term aurophilic attraction for intra- and intermolecular Au+–Au+ contacts was used.17 |
| ¶ Note added in proof: an Ag–Ag distance of 265.44(11) pm has been reported in [MeSi{SiMe2N(p-Tol)}3SnAg]2: B. Findeis, L. H. Gàde, I. J. Scowen and M. McPartlin, Inorg. Chem., 1997, 36, 960. |
| This journal is © The Royal Society of Chemistry 2003 |
