Novel gas-phase ion–molecule aromatic nucleophilic substitution in β-carbolines
Norberto P.
Lopes
ab,
Tatiana
Fonseca
a,
John P. G.
Wilkins
c,
James
Staunton
a and
Paul J.
Gates
*a
aDepartment of Chemistry, University of Cambridge, Lensfield Road, Cambridge, UK CB2 1EW. E-mail: pjg1002@cam.ac.uk; Fax: 01223 336362; Tel: 01223 336359
bFaculdade de Ciências Farmacêuticas de Ribeirão Preto, Universidade de São Paulo, Via do Café S/N, CEP 14.040-903, Ribeirão Preto-SP, Brazil
cUnilever Safety and Environmental Assurance Centre, Colworth House, Sharnbrook, Bedfordshire, UK MK44 1LQ
First published on 26th November 2002
Abstract
We report a novel gas-phase ion–molecule aromatic-nucleophilic substitution reaction between β-carbolines and water vapour, that accounts for the observation of ions with higher masses than the precursor ion in the MS/MS spectra.
β-Carbolines belong to a class of compounds, the heterocyclic aromatic amines (HAAs), that can be formed by the heating of protein rich food. They are proven mammalian carcinogens1,2 and legislative demands for the analysis of toxic residues in food has led to an increasing number of published methods for their detection.2 Previously published collision-induced dissociation (CID) mass spectra of harman3,4 show some bizarre ions occurring as [M
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H
H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1]+ or [M
1]+ or [M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H+
H+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2]+. Previously, no attempt has been made to explain the presence of these unusual ions.
2]+. Previously, no attempt has been made to explain the presence of these unusual ions.
Recently, the gas-phase reactivity of cyclic N-alkyl and N-acyliminium ions toward allyltrimethylsilane was compared with their eletrophilicity in solution. It was found that there was some correlation and that carbocation stability is a driving force in this reaction.5 Also, a previously published chemical ionisation study showed the possibility of gas-phase reactions occurring during MS analysis. A mechanism for gas-phase aromatic nitration by protonated methyl nitrate was described.6
Our preliminary accurate-mass analysis† performed on norharman (1, Fig. 1) also showed the presence of ions that were not the result of direct fragmentation of the parent ion. This suggested that other processes were occurring in the gas-phase besides the more usual neutral losses or carbocation rearrangements. The presence of these initially inexplicable ions in the spectra prompted the present investigation of gas-phase reactions occurring during MS/MS analysis. In an attempt to explain this new gas-phase chemistry, a series of HAAs (2–6; Fig. 1) were also analysed and the results compared to those already obtained for norharman 1 and the other previously published gas-phase chemistry.4,5
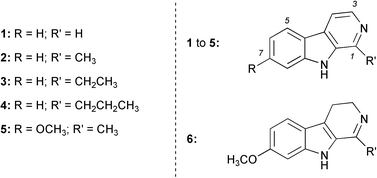 | ||
| Fig. 1 The β-carbolines. | ||
The spectrum of norharman 1 (C11H8N2, molecular mass 168) had a peak at higher m/z than the corresponding parent ion (Table 1). For example, an ion was observed at m/z 185. The exact mass difference between it and the [M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H]+ ion at m/z 169 corresponds to an oxygen atom. It is proposed that this is the result of addition of H2O followed by the elimination of H2. This process must be occurring after the isolation step in the ICR cell of the mass spectrometer and is presumed to be due to an ion–molecule reaction with residual water vapour present in the FT-ICR. This ion–molecule reaction is evidence that aromatic substitution in the gas-phase may happen to HAAs. The mechanism of the overall process is proposed in Scheme 1. The mechanism shows strong similarities to Chichibabin substitution of nitrogen hetreocyclic compounds.7 Chichibabin substitution occurs by an addition–elimination mechanism at the position α to the nitrogen in the pyridine ring.8 The mechanism proceeds via a similar process.
H]+ ion at m/z 169 corresponds to an oxygen atom. It is proposed that this is the result of addition of H2O followed by the elimination of H2. This process must be occurring after the isolation step in the ICR cell of the mass spectrometer and is presumed to be due to an ion–molecule reaction with residual water vapour present in the FT-ICR. This ion–molecule reaction is evidence that aromatic substitution in the gas-phase may happen to HAAs. The mechanism of the overall process is proposed in Scheme 1. The mechanism shows strong similarities to Chichibabin substitution of nitrogen hetreocyclic compounds.7 Chichibabin substitution occurs by an addition–elimination mechanism at the position α to the nitrogen in the pyridine ring.8 The mechanism proceeds via a similar process.
| Fragment | Formula | Relative intensity (%) | Observed mass |
|---|---|---|---|
| Norharman 1 | |||
| A | C11H9N2+ | 100 | 169.07597 (−0.30) |
| C or E | C11H9N2O+ | 35 | 185.07090 (−0.22) |
185![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
C11H7N2+ | 1 | 167.06039 (+0.12) |
| Harman 2 | |||
| A | C12H11N2+ | 100 | 183.09164 (−0.16) |
| C | C11H9N2O+ | 50 | 185.07093 (−0.05) |
C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
C11H7N2+ | 10 | 167.06040 (+0.18) |
| E | C12H11N2O+ | 5 | 199.08659 (0.00) |
E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
C12H9N2+ | 5 | 181.07597 (−0.28) |
| Compound 3 | |||
| A | C13H13N2+ | 100 | 197.10730 (−0.10) |
| C | C11H9N2O+ | 1 | 185.07083 (−0.59) |
C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
C11H7N2+ | 1 | 167.06040 (+0.18) |
| E | C13H13N2O+ | 35 | 213.10220 (−0.19) |
E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
C13H11N2+ | 20 | 195.09170 (+0.15) |
| Compound 4 | |||
| A | C14H15N2+ | 95 | 211.12283 (−0.66) |
| C | C11H9N2O+ | 1 | 185.07075 (−1.03) |
C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
C11H7N2+ | 1 | 167.06057 (+1.20) |
| E | C14H15N2O+ | 100 | 227.11792 (+0.13) |
E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
C14H13N2+ | 5 | 209.10744 (+0.57) |
| Harmine 5 | |||
| A | C13H13N2O+ | 100 | 213.10219 (−0.23) |
| C | C12H11N2O2+ | 1 | 215.08193 (+2.00) |
C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
C12H9N2O+ | 1 | 197.07074 (−1.01) |
| E | C13H13N2O2+ | 1 | 229.09720 (+0.22) |
E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) − −![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
C13H11N2O+ | 1 | 211.08628 (−1.47) |
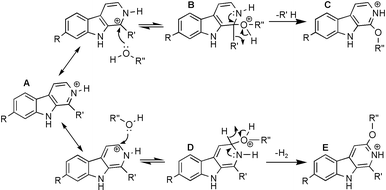 | ||
| Scheme 1 Proposed mechanism of nucleophilic substitution. | ||
Harman 2 (C12H10N2, molecular mass 182) has a methyl group in the 1 position (Fig. 1), was analysed to compare the regiochemistry of the possible aromatic substitution. Fig. 2 shows the MS/MS spectrum. For this compound, fragment E was observed at m/z 199 in addition to the parent ion and a fragment C at m/z 185 (Table 1) was also detected. The accurate mass difference between m/z 199 and 183 corresponds again to the addition of H2O and elimination of H2, but fragment C corresponds to the substitution of CH3 by OH resulting in CH4 elimination. Accurate-mass analysis also shows the presence of oxygen in the fragment at m/z 182 (Table 1) which is probably the loss of NH3 from ion C. These bizarre ions, [M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H
H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1]+ or [M
1]+ or [M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H
H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2]+, in the spectra of norhaman are accounted for by C and E, respectively. Both ions C and E lose water, further proving the presence of oxygen in these ions (see Table 1). The observed masses for these ions are all within 1 ppm of the theoretical masses—no other possible formula combinations are within 50 ppm.
2]+, in the spectra of norhaman are accounted for by C and E, respectively. Both ions C and E lose water, further proving the presence of oxygen in these ions (see Table 1). The observed masses for these ions are all within 1 ppm of the theoretical masses—no other possible formula combinations are within 50 ppm.
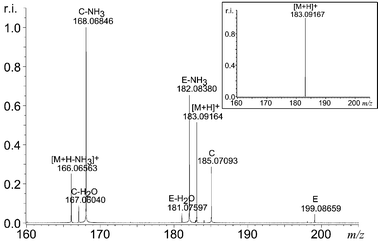 | ||
| Fig. 2 The MS/MS spectrum of harman (m/z 183). The insert is the isolation spectrum before MS/MS—this clearly shows that only m/z 183 is present in the ICR cell prior to the MS/MS step. | ||
Compounds 3 and 4 were prepared as described in the literature9 and analysed to eliminate the possible rearrangement processes and confirm the proposed CH4 elimination for harman 2. In the MS/MS analysis, performed under the same conditions no substitution was observed. However, in source dissociation, an ion corresponding to fragment E (m/z 213 and 227 respectively—see Table 1) was observed in the spectra of both compounds. Also the important fragment C was detected at m/z 185 (Table 1) confirming, as expected, the ethane and propane elimination. These results confirm that this reaction occurs with the same regiochemistry as with the Chichibabin substitution reaction.
The proposed mechanism for this aromatic substitution reaction implies a rearomatisation (intermediates B and D) and also the gain of one possible resonance structure by the addition of the OR group. To confirm this proposal, compounds 5 and 6 (Fig. 1) were also analysed. As expected, compound 5 showed fragments C and E and compound 6 showed no ions as a result of the substitution. These results are fully in agreement with the proposed mechanism and support the proposed intermediates B and D.
Finally, the cell of the FT-ICR was separately saturated with ethanol vapour and deuterated methanol vapour, to try to produce the addition–elimination products. In the first analysis, harman 2 with ethanol vapour, an ion at m/z 213 corresponding to ion C was produced, in agreement with the aromatic substitution mechanism proposed in Scheme 1. In the second analysis, harman 2 with deuterated methanol vapour, ions at m/z 202 and 203 were observed. Scheme 2 shows these two new ions F and G (Table 2) produced by the addition elimination of deuterated methanol and the deuterium exchange in the gas-phase. These fragments were the final proof for the proposed ion–molecule addition–elimination mechanism.
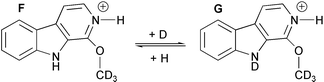 | ||
| Scheme 2 The production of ions F and G. | ||
| Fragment | Formula | Relaive intensity (%) | Observed mass |
|---|---|---|---|
| EtOH | |||
| A | C12H11N2+ | 100 | 183.0922 (+2.73) |
| C (R″ = H) | C11H9N2O+ | 100 | 185.0699 (−5.40) |
| C (R″ = Et) | C13H13N2O+ | 3 | 213.1019 (−1.41) |
| E (R′,R″ = H) | C12H11N2O+ | 8 | 199.0875 (−4.52) |
| CD3OD | |||
| A | C12H11N2+ | 100 | 183.0924 (+3.82) |
| C (R″ = H) | C11H9N2O+ | 5 | 185.0704 (−2.70) |
| F | C12H8D3N2O+ | 45 | 202.1054 (0.00) |
| G | C12H7D4N2O+ | 50 | 203.1115 (−0.98) |
In summary, we report here, the first evidence of a new gas-phase aromatic nucleophilic substitution of HAA ions during ESI-CID-MS/MS.
This work was supported by ‘Fundação de Amparo a Pesquisa do Estado’ (São Paulo, Brazil), ‘Biotechnology and Biological Sciences Research Council’ (London, UK) and the Unilever Safety and Environmental Assurance Centre (Bedfordshire, UK).
Notes and references
- P. Pais, C. P. Salmon, M. G. Knize and J. S. Felton, J. Agric. Food Chem., 1999, 47, 1098–1108 CrossRef CAS.
- E. Richling, D. Haring, M. Herderich and P. Schreier, Chromotographia, 1997, 48, 258–262 Search PubMed.
- P. Pais, E. Moyano, L. Puignou and M. T. Galceran, J. Chromotogr. A, 1997, 775, 125–136 Search PubMed.
- R. Richling, M. Herderich and P. Schreier, Chromotographia, 1996, 42, 7–11 Search PubMed.
- M. G. M. D’Oca, L. A. B. Moraes, R. A. Pilli and M. N. Eberlin, J. Org. Chem., 2001, 66, 3854–3864 CrossRef CAS.
- M. Aschi, M. Attinà, F. Cacace and A. Ricci, J. Am. Chem. Soc., 1996, 116, 9535–9542.
- W. W. Paudler and T. J. Kress, J. Org. Chem., 1968, 33, 1384–1387 CrossRef CAS.
- R. Foster and C. A. Fyfe, Tetrahedron, 1969, 25, 1489–1496 CrossRef CAS.
- H. R. Snyder, S. M. Parmerter and L. Katz, J. Am. Chem. Soc., 1948, 70, 222–225 CrossRef CAS.
Footnote |
† All the analyses were performed by high-resolution, accurate-mass electrospray ionisation on a Bruker Daltonics (Billerica, MA, USA) BioApex II 47e FT-ICR mass spectrometer. The [M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) + +![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H]+ ions were isolated to isotopic purity and submitted to CID-MS/MS (CO2 collision gas). H]+ ions were isolated to isotopic purity and submitted to CID-MS/MS (CO2 collision gas). |
| This journal is © The Royal Society of Chemistry 2003 |
