Dendrimers as scaffolds for the synthesis of spherical porphyrin arrays
Pablo
Ballester
b,
Rosa M.
Gomila
b,
Christopher A.
Hunter
a,
Amy S. H.
King
a and
Lance J.
Twyman
*a
aKrebs Institute for Biomolecular Science, Department of Chemistry, University of Sheffield, Sheffield, UK S3 7HF. E-mail: l.j.twyman@sheffield.ac.uk; Tel: 0114 2229560
bDepartemant de Química, Universitat de les Illes Balears, 07071, Palma de Mallorca, Spain
First published on 28th November 2002
Abstract
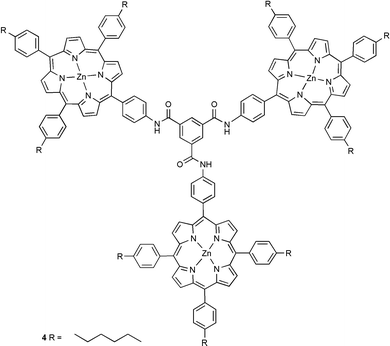
This communication describes a self assembled porphyrin sphere. The globular macromolecular assembly contains 12 terminal porphyrins and has a molecular mass in excess of 15000 g mol−1.
Nature′s most sophisticated solar energy storage systems, found in photosynthetic organisms, contain large numbers of porphyrins held in particular three-dimensional arrays. These complex assemblies capture photons and transfer them to a reaction center where they ultimately power cellular processes. Since their discovery a considerable amount of effort has been directed towards designing and synthesizing artificial structures capable of converting solar energy into a useable fuel.1 One particularly successful route towards these macromolecular structures has been to use supramolecular or non-covalent chemistry.2 These methods have the advantage of simplicity of synthesis, which in turn allows us to test structural modifications and correct any faults in design, without resynthesizing the complete macromolecule. In this paper, we report our initial efforts in this area, namely the self assembly of a large spherical array of porphyrins. Our general design involves a spherical ‘scaffold’ molecule capable of coordinating a large number of porphyrin units to its periphery.
Specifically, our design involved the pyridine terminated dendrimer 1, which has the potential to coordinate up to 16 zinc porphyrins to its periphery.3 Dendrimer 1 was synthesized from the commercially available DAB dendrimer 2 that possesses 16 terminal amine groups (Scheme 1).4 Reaction with an excess (2 equivalents per amine) of the activated ester of 4-pyridine carboxylic acid 35 and purification by aqueous extraction followed by precipitation, gave the desired pyridine terminated dendrimer in low yield.6 The 1H NMR spectrum of 1 showed all of the signals normally associated with DAB dendrimers, with additional signals at 7.75 ppm (32H, d) and 8.70 ppm (32H, d), which correspond to the 16 pyridine units. A peak corresponding to the molecular ion was also observed in the MALDI-TOF mass spectrum.
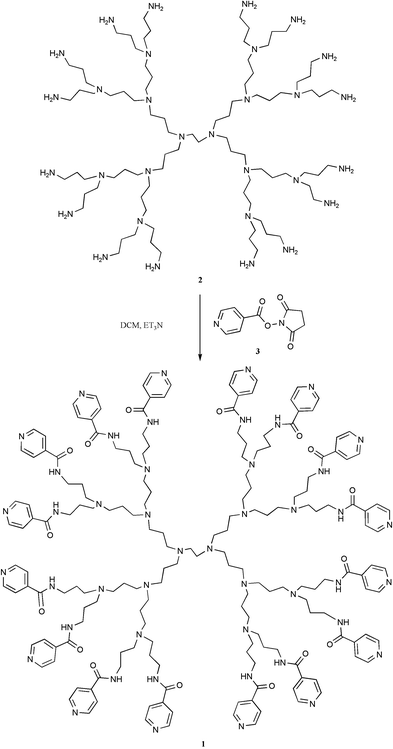 | ||
| Scheme 1 Synthesis of terminally functionalised dendrimer 1. | ||
Unfortunately, our initial attempts to form a porphyrin ball using tetraphenylporphyrin failed. The solubility of dendrimer 1 in all available non-coordinating solvents is lower than the μM level, so neither the UV/Vis absorption spectrum nor the 1H NMR spectrum of a mixture of tetraphenylporphyrin and dendrimer 1 could be recorded. A μM solution of a 16∶1 mixture of tetraphenylporphyrin and dendrimer 1 could be generated in 1% v/v methanol–dichloromethane. However at this concentration, the dendrimer–porphyrin coordination interactions are too weak to cause assembly (K ≈ 103 M−1), and the red shift of the porphyrin Soret band, characteristic of pyridine–zinc coordination, was not observed in the UV/Vis absorption spectrum.7 Binding to systems containing multiple interaction sites (such as the pyridine terminated dendrimer 1) can be improved if several ligands are tethered together. These improvements are often orders of magnitude greater than the additive affinities of the original monomeric ligands. Therefore, a zinc porphyrin oligomer with more interaction sites than the corresponding monomer should bind to the terminal pyridine moieties of 1 with a much higher affinity. If the cooperativity between the individual binding interactions is strong enough, such an oligomeric assembly should be able to withstand the presence of the competitive coordinating solvents required to dissolve dendrimer 1. Using this principle the porphyrin trimer 4 was prepared as a candidate terminal oligomeric ligand.8 The rigidity of trimer 4 should minimize entropic losses in cooperativity due to the restriction of conformational mobility. However, 4 is also sufficiently supple to allow distortion away from a planar geometry in order to optimize interactions with the convex surface of the dendrimer and ensure that all three porphyrins can be presented to the outer pyridine surface at the same time. A μM solution of porphyrin trimer 4 and dendrimer 1 (2∶1 with respect to individual porphyrin units and pyridine groups) was generated in 1% v/v methanol/dichloromethane. In this system, the UV/Vis absorption spectrum clearly indicated that zinc–pyridine complexation had occurred, as evidenced by the characteristic shift in the porphyrin Soret band (from 423 to 431 nm).9 In an effort to assess the stoichiometry of the complex, aliquots of the porphyrin trimer 4 (1.0 × 10−5 M in chloroform) were titrated into a cuvette containing 1.5 ml of a 4.25 × 10−7 M solution of dendrimer 1 in 1% v/v methanol–chloroform. Fig. 1 shows how the intensity of the porphyrin Soret band increases as a function of the amount of trimer added. The gradient clearly changes between 3.5 and 4.0 equivalents of porphyrin trimer 4 (the data for free and bound porphyrin show similar trends).
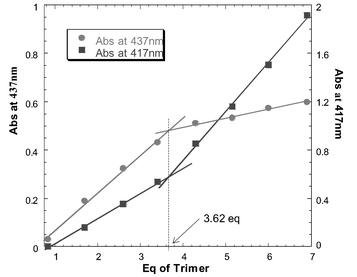 | ||
| Fig. 1 Titration plots of trimer 4 added to dendrimer 1 (following the absorbance for bound porphyrin at 437 nm and free porphyrin at 417 nm). | ||
During the first phase of the titration a porphyrin–dendrimer complex is formed, and once all of the accessible dendrimer sites are occupied, any additional porphyrin added remains uncomplexed. The change in gradient in the titration corresponds to approximately 11 porphyrins coordinated to the outer surface of the dendrimer. This is somewhat less than the theoretical maximum of 16 porphyrins, but presumably the trimer linker group is geometrically sub-optimal and sterically blocks access to some of the pyridine sites on the dendrimer surface. The value of 3.6 trimers coordinated to pyridine ligands suggests that after three trimers are bound to the surface of the dendrimer, there are no longer any sites that present three suitably orientated accessible pyridine ligands for tridentate complexation of another trimer. Therefore, the final trimer can only coordinate through two pyridine moieties, which still provide a cooperative binding interaction, and the final porphyrin is left unsatisfied. Thus the final product is a 4∶1 complex with 11 coordinated porphyrins and one dangling zinc porphyrin, which is not coordinated to a pyridine ligand.
In conclusion, our initial results described in this communication provide a unique example of a self assembled porphyrin sphere. The globular macromolecular assembly contains 12 terminal porphyrins (1 porphyrin remaining uncomplexed) and has a molecular mass in excess of 15300 g mol−1. The principles illustrated should provide a novel, general and simple methodology for the construction of spherical porphyrin arrays. Further extension of this methodology is currently being investigated in our laboratory.
The work was supported by grants from the BBSRC.
Notes and references
-
(a) J. P. Collin, A. Harriman, V. Heitz, F. Odobel and J. P. Sauvage, Coord. Chem. Rev., 1996, 148, 63–69 CrossRef CAS
; (b) A. Harriman and J. P. Sauvage, Chem. Soc. Rev., 1996, 25(1), 41–48 RSC
; (c) A. Adronov and J. M. J. Fréchet, Chem. Commun., 2000, 1701–1710 RSC
; (d) O. Mongin, A. Schuwey, M. A. Vallot and A. Gossauer, Tetrahedron Lett., 1999, 40, 8347–8350 CrossRef CAS
; (e) J. Li, J. R. Diers, J. Seth, S. I. Young, D. F. Bocian, D. Holten and J. S. Lindsey, J. Org. Chem., 1999, 64, 9090–9100 CrossRef CAS
; (f) H. S. Cho, N. W. Song, Y. H. Kim, S. C. Jeoung, S. Hahn and D. Kim, J. Phys. Chem., 2000, 104, 3287–3298 CrossRef CAS
; (g) A. K. Burrell, D. L. Officer, P. G. Plieger and D. C. W. Reid, Chem. Rev., 2001, 101, 2751–2796 CrossRef CAS
; (h) M. S. Choi, T. Aida, T. Yamazaki and I. Yamazaki, Angew. Chem., Int. Ed., 2001, 40, 3194–3198 CrossRef CAS
.
-
(a)
H. Ogoshi, T. Mizutani, T. Hayashi and Y. Kuroda, The Porphyrin Handbook, ed. K. M. Kadish, K. M. Smith and R. Guilard, Applications: Past, Present and Future, Academic Press, New York, 2000, vol. 6, pp. 279–340 Search PubMed
; (b) F. Odobel and J. P. Sauvage, New. J. Chem., 1994, 18, 1139 Search PubMed
; (c) C. A. Hunter and R. K. Hyde, Angew. Chem., Int. Ed. Engl., 1996, 35, 1936 CrossRef CAS
; (d) C. M. Drain, K. C. Russell and J. M. Lehn, Chem. Commun., 1996, 337 RSC
; (e) T. S. Balaban, A. Eichhöfer and J. M. Lehn, Eur. J. Org. Chem., 2000, 4047–4057 CrossRef CAS
; (f) A. K. Burrell, D. L. Officer, D. C. W. Reid and K. Y. Wilde, Angew. Chem., Int. Ed., 1998, 37, 114–117 CrossRef CAS
; (g) U. Michelsen and C. A. Hunter, Angew. Chem., Int. Ed., 2000, 39, 764–767 CrossRef CAS
; (h) K. Sugou, K. Sasaki, K. Kitajima, T. Iwaki and Y. Kuroda, J. Am. Chem. Soc., 2002, 124, 1182–183 CrossRef CAS
; (i) S. L. Darling, C. C. Mak, N. Bampos, N. Feeder, S. J. Teat and J. K. M. Sanders, New J. Chem., 1999, 23, 359–364 RSC
; (j) V. Balzani, S. Campagna, G. Denti, A. Juris, S. Serroni and M. Venturi, Sol. Energy Mater. Sol. Cells, 1995, 38, 159–173 CrossRef CAS
; (k) A. Ambrose, J. Li, L. Yu and J. S. Lindsey, J. Org. Chem., 2000, 2, 2563–2566
.
- J. F. G. A. Jansen and E. W. Meijer, J. Am. Chem. Soc., 1995, 117, 4417–4418 CrossRef CAS
.
-
(a) E. M. M. De Brabander-van den Berg and E. W. Meijer, Angew. Chem., Int. Ed. Engl., 1993, 32, 1308–1311 CrossRef
; (b) C. Wörner and R. Mulhaüpt, Angew. Chem., Int. Ed. Engl., 1993, 32, 1306–1308 CrossRef
; (c) A. W. Bosman, H. M. Janssen and E. W. Meijer, Chem. Rev., 1999, 99, 1665–1688 CrossRef CAS
.
- J. Tesler, K. A. Kruikschank and K. S. Schanze, J. Am. Chem. Soc., 1989, 11, 7221–7226
.
- L. J. Twyman and A. S. H. King, manuscript in preparation.
- To keep the dendrimer in solution at NMR concentrations, higher levels of methanol were required. Under these solvent conditions complexes could not be detected by NMR; presumably the increased concentration of methanol successfully competes with the porphyrins for binding.
- C. A. Hunter and R. M. Gomila, manuscript in preparation.
-
The Porphyrins, ed. David Dolphin, vol. 1: Structure and synthesis, Part A. Academic Press, New York–London, 1978 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2003 |