A simple, general and efficient ketone synthesis via alkylation and dephosphinoylation of β-keto-diphenylphosphine oxides†
David J.
Fox
,
Daniel Sejer
Pedersen
and
Stuart
Warren
*
University Chemical Laboratory, Lensfield Road, Cambridge, UK CB2 1EW. E-mail: sw134@cam.ac.uk
First published on 30th September 2004
Abstract
Products of difficult ketone alkylation reactions can be made selectively via activation with a diphenylphosphinoyl group; subsequent dephosphinoylation is easily achieved in base.
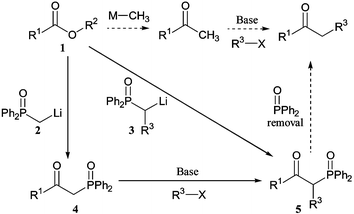
The synthesis of simple ketones by addition of organometallic reagents to carboxylic acid equivalents can be hampered by over-reaction to give tertiary alcohols. Additions of alkylmetal reagents to Weinreb amides1 do provide methods for selective ketone synthesis. Lithiated phosphine oxides 2 and 3, however add to simple alkyl esters 1 to give ketones 4 and 5 in high yield (Scheme 1). The β-ketophosphine oxides so formed are useful intermediates in the synthesis of (E)-olefins2 and cyclopropanes.3 In these syntheses, the phosphinoyl group is removed from the molecule by attack of an intramolecular alkoxide nucleophile.
 | ||
| Scheme 1 | ||
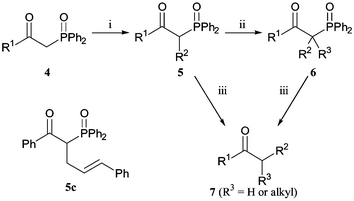
Current research projects within our group demanded the high-yielding synthesis of simple alkyl and aryl ketones via enolate alkylation, but it proved difficult to perform selective mono-alkylations of methyl ketones in standard conditions (Scheme 1). Related hydrazones could be alkylated instead and cleaved to give ketones,4 but as an alternative we hoped that β-ketophosphine oxides could be selectively alkylated and then dephosphinoylated in basic conditions with hydroxide as the intermolecular nucleophile. Alkylation of (diphenylphosphinoyl)methyl ketones 4 provides an alternative to the addition of more elaborate lithiated phosphine oxides 3 to esters.3
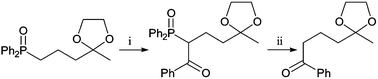
Initial investigations involved the treatment of ketone 5c
(R1
= Ph, R2
=
(E)-Ph(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)CH2–) with KOH in methanol at reflux. Dephosphinoylation was complete in a few hours and the desired product 7c was obtained in near quantitative yield (Table 3). To test the generality of this method, (diphenylphosphinoyl)methyl ketones 4
(R1
= Ph or Me) were alkylated with a range of electrophiles (Scheme 2, Table 1). If one equivalent of base and electrophile was used, only monoalkylation was observed. Treatment of mono-substituted ketones 5 in the same conditions allows for the introduction of a second, different alkyl group (Scheme 2, Table 2). Interestingly, a second alkylation is not possible for aryl ketones (R1
= Ph), even with heating. This is not currently understood. Base mediated dephosphinoylation occurs in good yield for a wide range of substrates 5 and 6
(Scheme 2, Table 3).
CH)CH2–) with KOH in methanol at reflux. Dephosphinoylation was complete in a few hours and the desired product 7c was obtained in near quantitative yield (Table 3). To test the generality of this method, (diphenylphosphinoyl)methyl ketones 4
(R1
= Ph or Me) were alkylated with a range of electrophiles (Scheme 2, Table 1). If one equivalent of base and electrophile was used, only monoalkylation was observed. Treatment of mono-substituted ketones 5 in the same conditions allows for the introduction of a second, different alkyl group (Scheme 2, Table 2). Interestingly, a second alkylation is not possible for aryl ketones (R1
= Ph), even with heating. This is not currently understood. Base mediated dephosphinoylation occurs in good yield for a wide range of substrates 5 and 6
(Scheme 2, Table 3).
| 4 |
Alkylating agent
R2-X |
Product (method)a | Yield (%) |
|---|---|---|---|
| R1 | |||
| a Alkylation conditions: a) NaOMe, R2-X, THF, 20 °C; b) NaOMe, R2-X, NaI, THF, 20 °C; c) NaH, R2-X, DMF. | |||
| Me | (E)-PhCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2Br CHCH2Br |
5a (a) | 87 |
| Me | PhCH2Br | 5b (a) | 72 |
| Ph | (E)-PhCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2Cl CHCH2Cl |
5c (b) | 97 |
| Ph | CH3(CH2)11I | 5d (c) | 71 |
| Ph | PhC(O)CH2Br | 5e (a) | 86 |
| Ph | (Z)-PhCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2Br CHCH2Br |
5f (a) | 85 |
| Starting material 5 |
Alkylating agent
R3-X |
Alkylation producta | Yield (%) | |
|---|---|---|---|---|
| R1 | R2 | |||
| a Alkylation conditions: NaOMe, R2-X, THF, 20 °C. | ||||
| Ph | Me | (E)-PhCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2Br CHCH2Br |
6a | 0 |
| Me | Me | (E)-PhCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2Br CHCH2Br |
6b | 97 |
| Et | Me | (E)-PhCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2Br CHCH2Br |
6c | 81 |
| i-Pr | Me | (E)-PhCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2Br CHCH2Br |
6d | 80 |
| (CH2)2Ph | (E)-PhCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2 CHCH2 |
(CH3)2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2Br CHCH2Br |
6e | 87 |
| Me | (E)-PhCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2 CHCH2 |
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2Br CHCH2Br |
6f | 54 |
| Starting material | Ketone product | Yield (%) | Conditions |
|---|---|---|---|
| a Conversion by NMR with Ph2P(O)OMe as the other product. | |||
| 5a | 7a | 92 | NaOH/H2O/EtOH/reflux |
| 5b | 7b | 86 | NaOH/H2O/EtOH/reflux |
| 5c | 7c | 96 | KOH/MeOH/reflux |
| 5d | 7d | 68 | NaOH/H2O/EtOH/reflux |
| 5e | 7e | 68 | NaOH/H2O/EtOH/reflux |
| 5f | 7f | 93 | NaOH/H2O/EtOH/reflux |
| 6b | 7g | 96 | NaOH/H2O/EtOH/reflux |
| 6c | 7h | 91 | NaOH/H2O/EtOH/reflux |
| 6d | 7i | 97 | NaOH/H2O/EtOH/reflux |
| 6e | 7j | 80 | NaOH/H2O/EtOH/reflux |
| 6f | 7k | 71 | NaOH/H2O/EtOH/reflux |
| 5a | 7a | 95 | K2CO3/H2O/EtOH/reflux |
| 5c | 7c | 95 | K2CO3/H2O/EtOH/reflux |
| 5c | 7c | 0 | KF/H2O/EtOH/reflux |
| 5c | 7c | >95%a | NaOMe/MeOH/reflux |
The addition to an ester and alkylation of the product at the activated methylene or methine carbon followed by hydrolytic removal of the activating group is reminiscent of a Claisen ester-condensation, alkylation and decarboxylation.5 Simple Claisen condensations are complicated by the synthesis of mixtures of cross-condensed and self-condensed β-keto-ester products, which are often difficult to separate and would give mixtures of ketones. The phosphine oxide mediated method avoids the use of a second carbonyl group, providing selective intermediate synthesis and alkylations, and a non-acidic ketone synthesis, ideal for products with acetal or ketal functionality (Scheme 3). One-pot reactions are possible with this methodology: lithiated phosphine oxide addition to esters with basic work-up provides ketones in good yield. Alternatively a selective mono-alkylation product can be dephosphinoylated without isolation in excellent combined yield (Scheme 4).
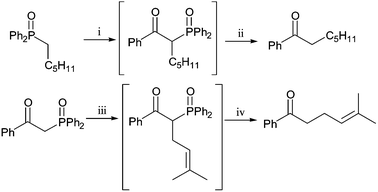 | ||
Scheme 4
Reagents and conditions: i, n-BuLi, −78 °C, PhCO2Me; ii, NaOH, H2O, EtOH, reflux, 66%
(2 steps); iii, NaOMe, (CH3)2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2Br, THF, 20 °C; iv, NaOH, H2O, EtOH, reflux, 89%
(2 steps). CHCH2Br, THF, 20 °C; iv, NaOH, H2O, EtOH, reflux, 89%
(2 steps). | ||
Dephosphinoylation of β-keto-phosphonates is possible with lithium aluminium hydride followed by highly acidic workup, but this method is not compatible with a range of common functional groups.6 Removal with base7 or mild acid8 has been reported only with highly activated substrates containing other significant electron-withdrawing groups. Standard keto-phosphine oxides, however, are nucleophilically cleaved using carbonate, but not fluoride. Sodium methoxide can be used as an alternative; this may be useful if methyl esters are present, avoiding unwanted hydrolysis (Table 3).
Alkylations of α,γ-dilithiated-β-ketophosphine oxides can also lead to the synthesis of interesting ketones.9 The treatment of variously substituted keto-phosphine oxides with two equivalents of LDA produces a dianion equivalent that selectively reacts at the less stabilised γ-position (Scheme 5). Systematic investigation into the effect on reaction of the substitution of phosphine oxide using cinnamyl bromide shows that increasing methylation at either the α- or γ-position reduced the yield of the γ-alkylation. These products can also be dephosphinoylated (Scheme 5). The same α- or γ-disubstituted keto-phosphine oxide can be produced with alkylation in either order. Comparison of the two routes to ketone 11 shows that both alkylation yields are higher if the γ-substituent is introduced first (Scheme 6).
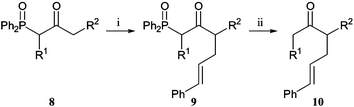 | ||
Scheme 5
Reagents and conditions: i, LDA (2 eq.), THF, −78 °C, (E)-PhCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2Br; ii, a, NaOH, H2O, EtOH, reflux, or b, KOH, MeOH, reflux (see Table 4). CHCH2Br; ii, a, NaOH, H2O, EtOH, reflux, or b, KOH, MeOH, reflux (see Table 4). | ||
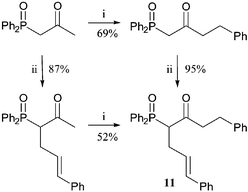 | ||
Scheme 6
Reagents and conditions: i, LDA (2 eq.), THF, −78 °C, BnBr; ii, NaOMe, (E)-PhCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2Br, THF. CHCH2Br, THF. | ||
Overall, selective activation of ketones towards regioselective alkylation with a removable diphenylphosphinoyl group is a general practical method with advantages over existing procedures.
DSP would like to thank the Alfred Benzon Foundation and the Leo Pharma Foundation for financial support.
Notes and references
- S. Nahm and S. M. Weinreb, Tetrahedron Lett., 1981, 22, 3815 CrossRef CAS.
- J. Clayden and S. Warren, Angew. Chem., Int. Ed. Engl., 1996, 35, 241 CrossRef CAS.
- A. Nelson and S. Warren, Tetrahedron Lett., 1996, 37, 1501 CrossRef CAS.
- E. J. Corey and D. Enders, Tetrahedron Lett., 1976, 11 CrossRef CAS; T. Cuvigny and H. Normant, Synthesis, 1977, 198 CrossRef CAS.
- A. P. Krapcho, Synthesis, 1982, 805 and 893 CrossRef CAS.
- J. E. Hong, W. S. Shin, W. B. Jang and D. Y. Oh, J. Org. Chem., 1996, 61, 2199 CrossRef CAS; S. Y. Lee, J. E. Hong, W. B. Jang and D. Y. Oh, Tetrahedron Lett., 1997, 38, 4567 CrossRef CAS; S. Y. Lee, C.-Y. Lee and D. Y. Oh, J. Org. Chem., 1999, 64, 7017 CrossRef CAS; S. Y. Lee, C.-Y. Lee and D. Y. Oh, J. Org. Chem., 2000, 65, 245 CrossRef CAS.
- A. Thenappan and D. J. Burton, Tetrahedron Lett., 1989, 30, 6113 CrossRef CAS; A. Thenappan and D. J. Burton, J. Org. Chem., 1991, 56, 273 CrossRef; S. R. Piettre, C. Girol and C. G. Schelcher, Tetrahedron Lett., 1996, 37, 4711 CrossRef CAS.
- D. Y. Kim, Synth. Commun., 2000, 30, 1205 CAS; D. Y. Kim, J. S. Choi and D. Y. Rhie, Synth. Commun., 1997, 27, 1097 CAS.
- R. S. Torr and S. Warren, J. Chem. Soc., Perkin Trans. 1, 1983, 1173 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: experimental section. See http://www.rsc.org/suppdata/cc/b4/b410144h/ |
| This journal is © The Royal Society of Chemistry 2004 |