Advances in organic field-effect transistors
Yanming Sun, Yunqi Liu* and Daoben Zhu*
Center for Molecular Science, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100080, China. E-mail: liuyq@mail.iccas.ac.cn
First published on 24th November 2004
Abstract
Since organic field-effect transistors (OFETs) were first described in 1987, they have undergone great progress, especially in the last several years. Nowadays, the performance of OFETs is similar to that of amorphous silicon (a-Si : H) devices and they have become one of the most important components of organic electronics. This feature article introduces briefly the operating principles, fabrication techniques of the transistors, and in particular highlights the recent progress, not only including materials and fabrication techniques, but also involving organic single crystal FETs and organic light-emitting FETs, which have been reported recently. Finally, the prospects and problems of OFETs that exist are discussed.
 Yanming Sun | Yanming Sun, born on January 2, 1979, graduated from the Department of Chemistry, Shandong University, China in July 2002. Since September 2002, he has been a PhD student at the Institute of Chemistry, Chinese Academy of Sciences (CAS). His research work includes preparation and characterization of organic field-effect transistors, and their potential applications. |
 Yunqi Liu | Yunqi Liu, born on April 1, 1949, graduated from the Department of Chemistry, Nanjing University in 1975, received a doctorate from Tokyo Institute of Technology, Japan in 1991. Presently, he is a professor of the Institute of Chemistry, CAS. His research interests include molecular materials and devices. |
 Daoben Zhu | Daoben Zhu, born on August 20, 1942, finished his graduate courses at the East China University of Science and Technology in 1968. Currently, he is a professor and Director of the Organic Solids Laboratory of the Institute of Chemistry, CAS. He was selected as an academician of CAS in 1997. His research interests include molecular materials and devices. |
1. Introduction
Organic field-effect transistors (OFETs), whose characteristics are modulated by an electrical field, are probably the most prominent constituents of modern microelectronics. Since they were first described in 1987,1 they have undergone great progress, especially in the last several years. OFETs have many advantages over conventional silicon technology: they can be fabricated at low cost, large area coverage and on flexible substrates.2–5 They are key building blocks for applications such as low-end display driving circuits and low-cost memory devices for smart cards and price tags etc.6–12 Additionally, organic integrated circuits have been demonstrated13–16 and all-polymer integrated circuits have been applied to commerce.17 Compared to the performance of FETs based on single-crystalline inorganic semiconductors, such as Si and Ge, the charge carrier mobility is about three orders of magnitude lower.18 However, recently, the mobility of OFETs based on rubrene single crystals was found to be 15.4 cm2 V−1 s−1,19 which exceeds that of amorphous silicon (a-Si : H) devices. As the pace of development of dimensions of inorganic transistors is rapidly approaching its limit, the OFETs exhibit tremendous potential for future application.Traditionally, the accepted view has been that organic materials are not electric and they have been widely used as insulators, until the 1970s, when Heeger et al. found that polyethylene molecules could become good conductors by doping.20 Since then the conducting polymers have received increasing attention from the research community and industry. Studies on organic semiconductors which include conjugated polymers, oligomers, or other molecules have greatly enhanced the development of OFETs. Nowadays, some OFETs can compete with amorphous silicon FETs, which are now preferred to conventional crystalline silicon FETs in applications where large areas are needed. For example, the entrenched technology in large area electronics applications, especially backplanes of active matrix liquid crystal pixels (AMLCDs), is based on FETs comprising hydrogenated amorphous silicon active layers. During the process of deposition a-Si : H a high processing temperature is required, which makes it impossible to fabricate an AMLCD based on such FETs on a transparent plastic substrate. However, OFET devices can be processed at or close to room temperature and thus are compatible with transparent plastic substrates.2 So, OFETs can compete directly with a-Si : H FETs and the performance is almost as good as that of a-Si : H devices.
In this feature article, we introduce the operating principles, fabrication techniques of OFETs, and in particular we highlight recent progress, not only including the materials and fabrication techniques, but also involving organic single crystal FETs and organic light-emitting FETs. Finally, the prospects and problems that exist are discussed.
2. Modeling of the electrical characteristics of OFETs
A typical FET is constructed with the basic components as shown in Fig. 1: source, drain and gate electrodes, a dielectric layer, and a semiconducting layer. OFETs adopt the architecture of the thin film transistor (TFT), which has proven its adaptability with low conductivity materials. The current flow between the drain and source electrodes is modulated by the applied gate voltage. When no gate voltage (VG) is applied, the drain current (ID) is very low if the semiconductor is not highly doped and the transistor is normally off. With an increase in the gate voltage, a layer of mobile charges can accumulate at the interface between the semiconductor and insulator. Then, the current is bigger due to the increased charge carriers and thus the transistor is in the on state. That is the operating principle of OFETs.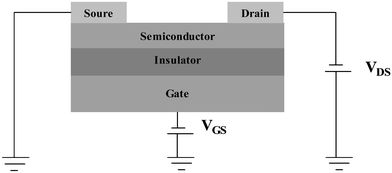 | ||
| Fig. 1 Schematic of device configuration of OFETs. | ||
Fig. 2 shows a typical output characteristic of an OFET, which corresponds to a device using copper phthalocyanine (CuPc) as the semiconductor, 500 nm thick SiO2 (Ci, 10 nF cm−2) modified with an octadecyltrichlorosilane (OTS) self-assembled monolayer (SAM) as the gate insulator, a heavily doped n-type Si wafer as the gate electrode and gold as the source and drain electrodes. When the gate electrode is biased negatively, the transistor based on CuPc operates in the accumulation mode and the holes are the major charge carriers in the transistor channel. From the output, we can see that with the drain voltage increase, the device gradually enters the saturation regime from the linear regime where the drain current becomes independent of the drain bias. The current ID modulated by VG is approximately determined from the following equations:
| ID = (W/L)Ciμ(VG − VT)VD (linear regime) | (1) |
| ID = (W/2L)Ciμ(VG − VT)2 (saturation regime) | (2) |
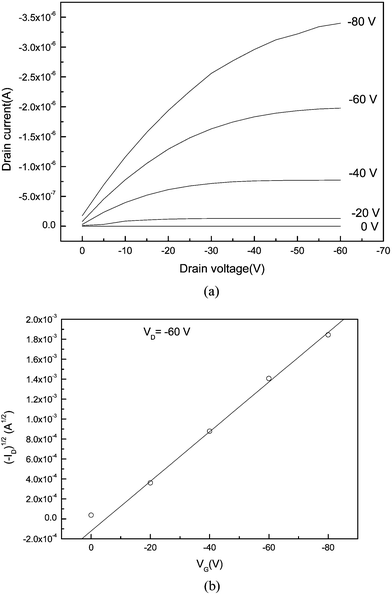 | ||
| Fig. 2 (a) Output characteristic of an OFET comprising a polycrystalline copper phthalocyanine thin film, 500 nm thick SiO2 insulator layer modified with an OTS SAM, a heavily doped n-type Si wafer as the gate electrode and Au as the source and drain electrodes. (b) Drain current versus gate-source voltage characteristics of OFET in the saturation regime at a drain–source voltage VD of −60 V. | ||
There are several parameters in characterizing an OFET, such as the field-effect mobility, an on/off ratio, threshold voltage and subthreshold swing. The field-effect mobility quantifies the average charge carrier drift velocity per unit electric field, whereas the on/off ratio is defined as the drain-source current ratio between the on and off states. The threshold voltage VT is a parameter that evaluates the amount of traps. The subthreshold S is a measure of how rapidly the devices switches from the off state to the on state in the region of exponential current increase and is defined by S = ∂VG/∂(logId).
In the saturation regime, we often used eqn. (2) to estimate the charge carrier mobility (μ). From the slope of the plot of (ID)1/2versusVG, μ can be calculated. For the example shown in Fig. 2, the mobility is 1.8 × 10−3 cm2 V−1 s−1, the on/off ratio is 2.5 × 103 and the threshold voltage VT = 3 V. The subthreshold S is 13 V (decade)−1.
If organics are to compete with amorphous silicon circuits used to drive LC displays, the organic semiconductor should provide a field-effect mobility of ∼1 cm2 V−1 s−1, on/off ratios in the range of 106 and a threshold voltage near 0 V. However, for most of the organic semiconductors, the performances are below these criteria.
3. Conduction mechanism in organic semiconductors
In organic semiconductor materials, the molecules are kept together mainly by weak van der Waal's forces, which differ from the forces in inorganic semiconductors in which the atoms held together with very strong covalent bonds. When a large number of individual atoms are gathered together in a three dimensional lattice, the discrete atomic levels widen into bands and the charges move freely in delocalized bands with very high mobilities.2 The forces in the semiconductors determine the different charge transport. In inorganic semiconductors, charge transport occurs in delocalized states which are limited by the scattering of the carriers, mainly on phonons, that is, thermally induced lattice deformations. In this case, the mobility is limited by phonons that scatter the carriers and thus it is reduced as the temperature increases.2 In organic materials, the transport differs from the band transport of inorganic semiconductors. Currently, there is a general agreement that it occurs via polaron (the deformation of the lattice around the electron or hole) hopping between localized states.2,3 In this model, charge transport occurs by hopping of charges between localized scatterers and carriers are scattered at every step. Hopping is assisted by phonons and the charge mobility increases with temperature in organic semiconductors. According to this model, small polarons move via thermally activated hopping, resulting in a simple activated dependence of the mobility with temperature. When the temperature is in the appropriate temperature range, normally above 100 K, the activation energy of the organic semiconductors is high.9 In this temperature range, the charge mobility is in good agreement with the small polar model and the mobility is temperature dependent. At low temperatures, the activation energy is substantially reduced and the mobility is practically temperature independent or the mobility rises contrary to expectation. Accordingly, some people suggest band transport becomes the mechanism of charge carrying at low temperatures; however, we note that, because of the narrow effective width of the electron and hole bands, delocalized transport is unlikely in polycrystalline materials, where the evaluated mean-free path of carriers is shorter than the separation of two molecules, which renders the concept of band transport impossible. In some cases temperature independent mobility has been observed,5 even in thin films of pentacene. For example, Nelson and co-workers reported devices based on pentacene with a temperature independent mobility from 10 K up to room temperature.21 A simple thermally activated hopping transport model does not apply to that. It is possible for traps to make the mobility temperature independent. During thermal evaporation of pentacene, dissociations could be created, which act as traps to make the mobility temperature independent. It is also probably due to the device structure effects. For the device used, with source and drain contacts on the side of the active layer, there is a large concentration of traps close to the Au contact which dominates the carrier transport at low temperatures. The microscopic transport mechanism is still ambiguous.A preliminary model has been developed by the Thiais group, based on the multiple trapping and release (MTR) model.3,22,23 In this model, the field-effect mobility is gate bias dependent. When the gate bias is increased, the Fermi level gradually approaches the nearest delocalized band edge. In amorphous silicon, there exists near the delocalized bands an important density of localized levels, which act as traps for charge carriers. At low gate bias, nearly all induced charges go to the localized levels, where their mobility is very low. With an increase of the gate voltage, the Fermi level approaches the delocalized band and more traps are filled, which leads to an increase of the concentration of mobile carriers in the delocalized levels. As a result, the effective mobility increases. They have used this model to rationalize the characteristics of sexithiophene (6T-) and dihexylsexithiophene (DH6T-) based OFETs.
Although several models have been made such as the polaron hopping, MTR model24 and others, a temperature independent mobility can not be explained by available theories on charge transport in solids. Much work needs to be done to understand charge transport in organic semiconductors.
4. Organic semiconductors
Currently, π-conjugated organic oligomers and polymers are the subject of considerable current research interest in organic semiconductors. As the different charge carriers, the organic semiconductor can function either as p-type or n-type. In p-type semiconductors the majority carriers are holes, while in n-type semiconductors, the majority carriers are electrons. Accordingly, the transistors are p-type transistors or n-type transistors. The development of organic light-emitting diodes has introduced the concept of hole and electron transporting materials, which seems more pertinent for organic semiconductors.3 n-Type and p-type materials are mainly characterized by their high electron affinity and low ionization potential, respectively. However, most of the organic semiconductors investigated so far are p-type in their non-intentionally doped form. This is mainly because p-type semiconductors are stable in air and have relatively high mobility when they are used in OFETs. Unlike p-type semiconductors, most n-type semiconductors are sensitive to air and moisture,25 due to the organic anions, in particular carbanions, that react with oxygen and water under operating conditions. Furthermore, the n-type semiconductors have relatively low field-effect mobilities. But n-type semiconductors are important components in organic electronics. The aspect of development should have received much attention. Fig. 3 shows the chemical structures of several common organic semiconductors. In the next sections, we will discuss p-type semiconductors and n-type semiconductors, respectively.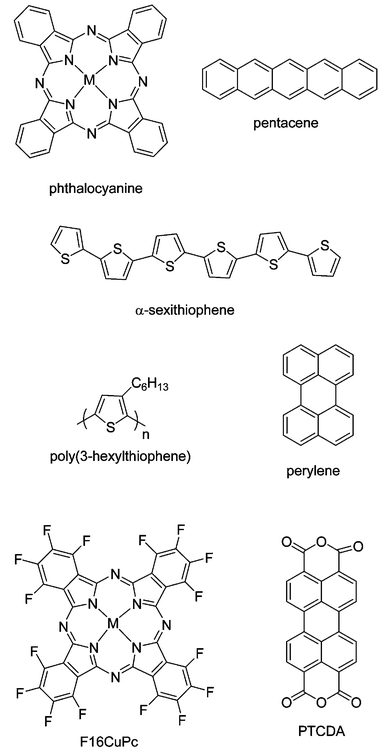 | ||
| Fig. 3 Chemical structures of several common organic semiconductors. | ||
4.1 p-Type semiconductors
Most organic materials tend to transport holes better than electrons, thus there are more p-type materials and most work has focused on them. The p-type semiconductors mainly consist of oligomers, pentacene, phthalocyanine, etc. Small molecules such as pentacene, α-6T etc. possess the best electronic characteristics to date.Pentacene is an aromatic compound with five condensed benzene rings and has been widely studied as a p-type semiconductor for OFETs. Its characteristics have been studied since the 1970s. The highest field-effect mobilities so far have been recorded for pentacene (0.3–0.7 cm2 V−1 s−1 on SiO2/Si substrates,26 1.5 cm2 V−1 s−1 on chemically modified SiO2/Si substrates,27 and 3 cm2 V−1 s−1 on polymer gate dielectrics28).
Current research on pentacene is mainly on its polycrystalline thin film due to its poor solubility. The fabrication techniques are mainly dependent on vacuum evaporation. Their characteristics have been depicted in many reviews,2,3 Here, we mainly discuss its soluble precursors or its derivatives. Recently, much work focused on using the materials as a soluble precursor allowing the fabrication of solution-cast unsubstituted pentacene.29–32 The soluble precursor molecules can be converted to pentacene upon heating. The mobilities of the precursors initially reported are very low. Recently, however, the performance of pentacene precursors has greatly improved. Müllen and co-workers reported a mobility of 0.2 m2 V−1 s−1 and an on/off ratio more than 106 were achieved by the chemically modified substrate and by optimization of the processing and conversion conditions of the precursor.30 Afzali et al. have developed a one-step synthesis of a soluble pentacene precursor that reverts to pentacene at moderate temperatures while retaining transport properties comparable to those of vacuum-deposited pentacene films.31 The precursor film was fabricated by spin-coating a chloroform solution on the substrate followed by annealing at 200 °C for 1.5 min or at 130 °C for 25 min under a nitrogen atmosphere to convert to pentacene. The highest mobility exhibited in the saturation regime was 0.89 m2 V−1 s−1. They also synthesized a photosensitive pentacene precursor using photosensitive methacrylamides as the starting material.32 Thin films of the precursor upon exposure to UV irradiation and final annealing at 150 °C can convert to pentacene films. There are also many studies on functionalized pentacene to improve π-orbital overlap for pentacene derivatives.33,34 The work is scarce and the study of pentacene is still in research.
Oligomers consisting of conjugated oligothiophene and polymers are promising charge transport semiconductors. The ease of chemical modification of their structures can allow us to fine-tune their properties. Conjugated oligothiophenes commonly have better solubilities and are easily purified, and thin films can be obtained with different methods. The charge carrier mobility can be improved by adding alkyl chains to the end of the oligothiophene rings.35,36 This probably enhances the π-orbital overlap due to the influence of the alkyl chains and improves the order of the film. In particular, α-sexithiophene (α-6T) and its derivatives have dominated as active organic materials.37–39 Carrier mobilities reported for α-6T OFETs have improved from 10−4 cm2 V−1 s−1 to greater than 0.01 cm2 V−1 s−1.40–42 Substituting the alkyl chains of the α-6T molecule led to an increase in carrier mobility to 0.13 cm2 V−1 s−1.36,43 Carrier mobilities near 0.2 cm2 V−1 s−1 have been reported for α-octithiophene OFETs with films deposited at 150 °C and higher.44 Recently, Halik and co-workers reported a carrier mobility of 1.1 cm2 V−1 s−1 obtained for alkyl-substituted oligothiophene.45 They synthesized and evaluated a series of alkyl-substituted oligothiophenes with different alkyl side chains lengths and different chromophore lengths ranging from four to six thiophene units. They found that the OFETs performance depended critically on the length of the side chains. The highest mobility was found for α,α′-diethylsexithiophene which was mainly due to the shorter side chains forming a significantly thinner barrier between the conjugated backbones, leading to more efficient carrier injection.
Polymers are attractive for OFETs because thin films of these materials can be obtained through simple solution techniques such as drop casting, spin coating and ink printing etc. But the mobilities are usually lower than the small molecules due to the poor molecular ordering and low crystallinity obtained by the solution techniques. However, mobilities beyond 0.1 cm2 V−1 s−1 have been reported with regioregular polythiophene with structure optimization or annealing etc.46,47 Another way to increase the performance of the polymers is through doping, but at the same time the conductivity increases and results in a high drain current at zero gate voltage. Ease of manufacturing, excellent physical properties and low cost play an important role in organic electronics.48,49
Phthalocyanines (Pcs) are the organic materials that have been studied most in organic semiconductors. Their excellent photoelectric characteristics have attracted enormous interest.50,51 Especially, the devices can exist in air for months due to their better thermal and chemical stability. They have been widely used as solar cells, optical limiters, and photoconductors.52,53 However, the charge mobilities of these compounds are low. The charge mobility can be up to 0.11 cm2 V−1 s−1 for OFETs having source/drain electrodes sandwiched between two layers of metallophthalocyanines.54
From the theoretical point of view, the transfer integral between neighboring molecules is the basic parameter to determine the charge mobilities. According to this guide, compounds with rigid, fused-ring structures are of interest for OFETs where strong π–π interactions are enhanced between adjacent molecules. Currently, a lot of fused aromatic compounds have been successfully synthesized with high mobilities, for example, dibenzothienobisbenzodithiophene (0.2 cm2 V−1 s−1),55 bisdithienothiophene (0.05 cm2 V−1 s−1),56 dihydrodiazapentacene (0.006 cm2 V−1 s−1),57 diphenylbenzo-dichalcogenophenes (0.17 cm2 V−1 s−1).58
4.2 n-Type semiconductors
Although most of the work to date has focused on p-type organic materials, high performance n-type semiconductors are important components of p–n junction diodes, bipolar transistors, and complementary circuits. But most of the n-type materials are unstable in air. The electron mobility has degraded or there is no field effect when the devices are exposed to air. Another reason for n-type OFET materials lagging behind p-type materials is that the metals used for making contact to organic semiconductors have work functions better suited for injection of holes into the HOMO than of electrons into the LUMO, which associates with the band levels of the organic materials. For most p-type materials, the ionization potentials are about 5 eV, which is near the metals' work functions. Low-work-function metals such as Al, Ca, Mg, usually oxidize easily and readily form reactive complexes with the organic semiconductor.59 Much effort should be guided towards the preparation of stable and high field-effect mobility n-type semiconductors.As we mentioned above, in order to obtain a material to transport electrons, it needs to have an accessible LUMO level for electron injection. For FET operation, electrons are conveniently injected into compounds with ionization potentials approximately 4 eV below vacuum.60 Higher potentials for reduction would make doping much more easy. By adding strong electron-withdrawing groups such as –F, –CN, and –Cl to the outer rings of molecules, good candidates for n-type semiconductors may be created. For example, Bao et al. reported on the use of F16CuPc as a novel n-type semiconductor. The hexadecahalogenated metallophthalocyanines were found to function as air-stable n-type semiconductors with a maximum electron mobility of 0.03 cm2 V−1 s−1.61 This work presents an interesting design rule in which known p-type semiconductors can be converted to n-type semiconductors by using the above-mentioned method.
Recently, Facchetti et al. explored a variety of the fluoroarene-modified thiophene semiconductors.62 Electron-deficient perfluoroarene substitution in the different positions of the thiophene rings can greatly affect the performance of semiconductors such as the molecular orbital energies, molecular packing etc. They found n- versus p-type transport may be influenced by superior core screening by the end-fluoroarene groups against environmental electron traps (O2, H2O), probably at the grain boundaries; other compounds without the end-fluoroarene groups still exhibited p-type semiconductivity. The electron mobility is 0.08 cm2 V−1 s−1 which is the highest mobility yet reported in the thiophene series. In their early work, they explored fluorohexylsexithiophene (DHF-6T) as the n-type material. Under a nitrogen atmosphere, mobilities of 0.02 cm2 V−1 s−1 and an on/off ratio of 105 could be obtained in the saturation regime using gold as the electrodes.63
Previously, Sakamoto et al. demonstrated that aromatic perfluorocarbons such as perfluoro-p-sexiphenyl (C36F26) were efficient n-type semiconductors for the electron-transport layer of organic light-emitting diodes.64 Recently, they have designed perfluoropentacene (C22F14) as a potential n-type semiconductor for OFETs.65 With this perfluoropentacene, they have fabricated OFETs, and bipolar OFETs and complementary circuits with pentacene. The field-effect mobility calculated in the saturation regime is 0.11 cm2 V−1 s−1 (on/off ratio = 105). Bipolar OFETs with the second layer of pentacene formed on the perfluoropentacene layer using gold electrodes. The field-effect mobility is 0.024 cm2 V−1 s−1 for the n-channel operation and 0.035 cm2 V−1 s−1 for the p-channel operation.
Another class of n-type materials researched early is perylene and its derivatives such as 3,4,9,10-perylenetetracarboxylic dianhydride (PTCDA), 3,4,9,10-perylenetetracarboxylic diimide (PTCDI) and their derivatives.66–68 However, these materials commonly have lower mobilities, but high mobilities are found with substituted PTCDA. Malenfant et al. reported that OFETs based on N,N′-dioctyl-3,4,9,10-perylenetetracarboxylic diimide (PTCDI-C8) as the organic semiconductor provided bottom contact devices with mobilities as high as 0.6 cm2 V−1 s−1 in the saturation regime and current on/off ratios >105.69
Chesterfield et al. have recently reported on the electrical characterization of the n-type organic material, 3′,4′-dibutyl-5,5″-bis(dicyanomethylene)-5,5″-dihydro-2,2′:5′,2″-terthiophene (DCMT).70,71 Mobility as high as 0.2 cm2 V−1 s−1 is observed by controlling the growth of DCMT films. When the films were grown at elevated substrate temperature, the material exhibited both n-type and p-type conduction. Below that temperature, the film only showed n-type behavior; however, the hole and electron mobilities in these devices were less than 10−4 cm2 V−1 s−1. This is an example of ambipolar transport in a thin-film transistor based on a single conjugated organic semiconductor material. Ambipolar device operation has been reported in two organic active layers in the same transistor previously.72
Reports on n-type semiconducting polymers for OFET applications are scarce. Most of the work on semiconducting polymers has focused on p-type materials, such as poly(3-hexylthiophene). However, Babel and Jenehke have reported the observation of field-effect electron mobility as high as 0.1 cm2 V−1 s−1 in a solution spin-coated conjugated ladder polymer, poly(benzobisimidazobenzophenanthroline) (BBL).73 This work demonstrates that electron transport can be as facile as hole transport in conjugated polymer semiconductors. However, the literature on n-type polymer materials remains sparse.
4.3 Several factors affecting the performances of OFETs
Traditional organic semiconductors used for OFETs commonly have high mobility and low intrinsic conductivity. High mobility can enhance the performance of devices and low intrinsic conductivity can deduce the drain current, improving the on/off ratio. Therefore, when designing a novel organic semiconductor, for both n-type and p-type semiconductors the rules above should be considered. On the one hand, the conduction band (LUMO or HOMO level) should be optimized in order to make the injection of charges from electrodes easy, on the other hand, the morphological characteristics should be also taken into account because the crystal packing of the materials greatly affects the transport properties.Firstly, the presence of impurities plays an important role in the characteristics of devices. In OFET devices, the carrier accumulation layer is occurring in the first few monolayers of the organic semiconductor at the interface with the insulator. Therefore, if the materials are impure, traps which alter the relative energy levels and inhibit the flow of charge carriers would be formed at the interface and in the interior of materials. Thus, impurities can greatly affect the mobility and the on/off ratio. If the materials are impure, impurities can function as dopants, thereby increasing the conductivity of the film, resulting in large leak currents and leading to a low on/off ratio. The importance of impurities for the limitations in device performance has been emphasized during the last few years. For example, recently, Jurchescu and co-workers have reported a mobility of μ = 58 cm2 V−1 s−1 at 225 K for purified pentacene single crystals.74 The crystals were obtained by vapor transport growth in argon flow after purification of the materials by a vacuum sublimation technique. The number of traps is reduced by two orders of magnitude compared with conventional methods.
Furthermore, there are many restrictions for organic materials used for OFETs. First, it should have a highly conjugated system which is rich in π-electrons. Second, it also needs good solubility and stability. As we know, the intrinsic carrier mobility depends critically on the degree of molecular ordering and on the extent of the π–π stacking in the materials. Commonly, a high mobility can be obtained with the mobile charge direction parallel to the direction of π-orbital overlap. For organic materials such as pentacene and phthalocyanine etc., the molecular planes are oriented perpendicular or approximately perpendicular to the substrate surface (see Fig. 4) and in that case, the carriers can transfer at a high rate. If the molecules are lying flat on the surface of the dielectric, the carriers would transport in the direction perpendicular to the π-orbital overlap leading to low mobilities. For example, the poor transport characteristics for PTCDA with mobilities of 10−5 to 10−4 cm2 V−1 s−1 were obtained. X-Ray diffraction measurements suggest that such low mobilities are mainly due to the PTCDA molecules stacking in planes nearly parallel to the substrate. For PTCDI-C8, electron mobility up to 0.6 cm2 V−1 s−1 was obtained. As a result, the long axis of PTCDI-C8 is expected to pack highly inclined to the substrate surface whereas the π-stacking direction is parallel to the surface, thus resulting in good mobility characteristics. However, how the structural and electronic factors such as the molecular structures, crystal packing etc. that are favorable for transport are still under investigation.
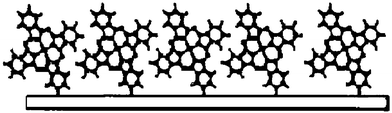 | ||
| Fig. 4 Organic molecular planes oriented approximately perpendicular to the substrate surface. | ||
The high mobility in pentacene molecules has been attributed to their ability to pack into well-organized polycrystalline films. In the solid state, the molecules are fully planar and pack along parallel layers and form the so-called herringbone packing in the pentacene crystal structure. The deposition conditions can affect the orders of thin films, which are reflected in the electronic properties.75,76 When the substrate temperature is kept close to −196 °C during deposition, the amorphous films grow, which is due to the disordered molecules in the solid. When the temperature is kept at room temperature, a highly ordered film is deposited and a mobility of 0.6 cm2 V−1 s−1 can be obtained.
The deposition conditions also affect the morphology of the organic materials. In our laboratory, we systemically study the influence of the substrate temperature of evaporation on the film characteristics of copper phthalocyanine.77Fig. 5 shows the transmission electron micrographs from CuPc films at different substrate temperatures. The morphologies differ greatly at different temperatures. The film deposited at room temperature is made of homogeneous small crystal grains. With increasing deposition temperature, the morphology of the films gradually changes from grains to rod-like and large flat crystals. Clearly, larger, more perfect flat crystals are far more preferable for carrier flow. However, nucleation at high substrate temperature is very sparse so that the resulting large and regular crystals end up being separated far from each other with severe film discontinuities and large gaps, which have a negative effect on the mobility of OFET devices. When the substrate temperature for deposition of CuPc is 120 °C, a mobility of 3.57 × 10−3 cm2 V−1 s−1 can be obtained. These results confirm that control of the substrate temperature allows us to monitor the grain size and shape together with homogeneity of structural organization.
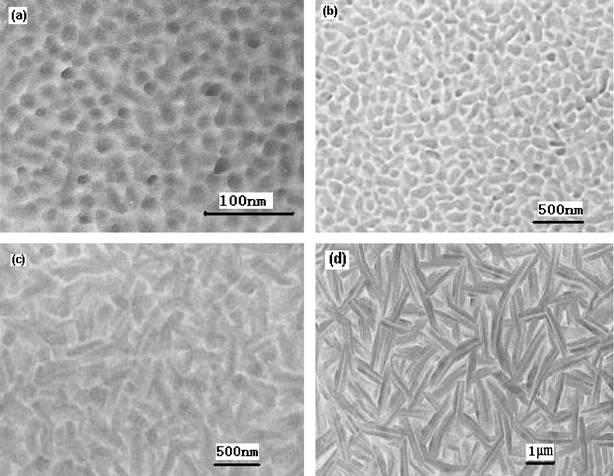 | ||
| Fig. 5 Transmission electron micrographs from CuPc films at different substrate temperatures. (a) Tsub = 20 °C, (b) Tsub = 120 °C, (c) Tsub = 170 °C, (d) Tsub = 200 °C. | ||
Other than the factors we mentioned above, there are many factors that probably affect the OFETs performances, such as different fabrication techniques, and the type of crystal packing (herringbone or π-stacking) etc. These factors can all have an influence on the OFETs performance. Our knowledge of these factor is insufficient to interpret all questions.
5. Fabrication techniques of OFETs
Recently, OFETs have achieved great progress in materials and fabrication techniques. A mobility of 15.4 cm2 V−1 s−1 was reported for rubrene single crystals and 1.5 cm2 V−1 s−1 for pentacene polycrystalline thin films. The performances of organic semiconductors obtained are combined with progress of the fabrication techniques. Conventional techniques such as photolithography, evaporation etc. due to expense or sophisticated devices are improved and substituted. Spin-coating, casting and printing techniques with low price have emerged. These techniques greatly promote the development of OFETs and become promising results for organic optical and electronic devices.OFETs consist of different layers of thin films, so interfaces between the layers are important factors that affect the transistor characteristics. By optimizing the semiconductor deposition process, controlling the substrate temperature, modifying the surface of the insulator with organics forming self-assembled monolayers on it, the performances can be greatly improved. Good contact of layers can not only enhance the ordering of molecules, minimizing the defects, but also facilitate the injecting of charges and transfer in the conducting channel.
Currently, the common fabrication techniques used include vacuum evaporation, Langmuir–Blodgett, solution-processed deposition and stamping or microcontact printing (μCP) techniques etc.
Vacuum evaporation
Vacuum evaporation techniques are some of the most used techniques currently. Organic semiconductor films can be deposited by sublimation in a chamber under high vacuum or ultrahigh vacuum. By controlling the substrate temperature and the deposition rate, highly ordered thin films with different thicknesses can be obtained. The purity of the organic materials is also an important factor. The mobility of OFETs produced by this technique is one or two orders of magnitude higher than those formed from solution-processed deposition techniques. Thin films of organic small molecules such as pentacene, phthalocyanine, oligothiophene are formed using this technique. However, its primary drawback is that this technique requires sophisticated instrumentation and can not be used for polymers.Langmuir–Blodgett
A Langmuir–Blodgett (LB) thin film is a kind of ultrathin and well-ordered thin film. The Langmuir–Blodgett technique is a technique which allows fine control of both the structure and thickness of the film at the molecular level.78 It is a conventional vertical dipping technique. Using this technique, single molecular monolayers with nearly no defects can be obtained. However, this technique is in principle restricted to amphiphilic molecules, composed of a hydrophobic chain and a hydrophobic headgroup, which is not the case for most of the materials used in OFETs.Solution-processed deposition
One of the most elegant ways employed for depositing polymers is spin-coating. By dropping or casting solutions to a certain rate of substrate, thin films can be obtained when the solvents evaporate. This technique requires organics with good solubility and the thickness of the thin films is only controlled by adjusting the concentration of the solution or the rate of the device. The emergence of the technique greatly reduces the price of OFETs and promotes the development of OFETs.Microcontact printing (μCP) techniques
The development of OFETs is combined tightly with the progress of microfabrication such as photolithography, soft lithography, etc.79–83 In conventional silicon devices, photolithography is the most used technology in microfabrication. However, this method is relatively expensive for low-end devices and is not suitable for organic materials because solvents or aqueous chemicals might contaminate the organic thin films. Within soft lithography, the μCP technique is one of the components which uses a soft stamp to form patterned microstructures of self-assembled monolayers on the surface of the substrate. By the μCP technique, lateral dimensions of about 30 nm to 500 µm can be obtained.84 Therefore, this method is rapidly becoming important for micropatterning and is very attractive for the electronics industry.Fig. 6 shows the schematic illustration of procedures for μCP. An elastomeric stamp is used to transfer molecules of the “ink” to the surface of the substrate by contact. An elastomeric stamp is usually prepared by replica molding by casting a liquid prepolymer of an elastomer against a master that has a patterned relief structure in its surface.84 Poly(dimethylsiloxane) (PDMS) is the most used elastomer. After printing, a different SAM can be formed on the underivatized regions by washing the patterned substrate with a dilute solution containing the second molecule. The components of the surface of the substrate can be metal (Au), polymer, and other organics. μCP is attractive because it is simple, inexpensive and flexible. It is suitable for forming patterns over large areas in a single process. The electrical performance of OFETs using the microcontact printing technique is similar to or better than those of devices fabricated using conventional lithography. However, for μCP, there are a number challenges that remain to be resolved. For example, many molecular inks used in microcontact printing are inclined to surface diffusion or edge disorder and μCP can only be used in SAM systems.85–87
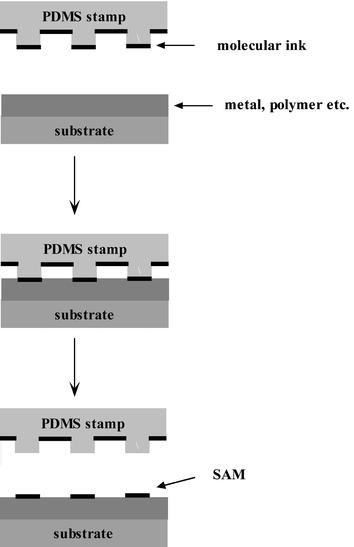 | ||
| Fig. 6 Schematic illustration of procedures of the microcontact printing process. | ||
6. Advances in OFETs
6.1 Advances in materials
Fig. 7 shows several high-performance organic semiconductors which have been reported recently and Table 1 shows the characteristics of some organic semiconductors used for OFETs. From this we can see that OFETs have been greatly improved in the past several years and can rival the performance of a-Si : H devices. Especially, the emergence of organic light-emitting FETs and organic single crystal FETs promotes the OFETs to a new development phase.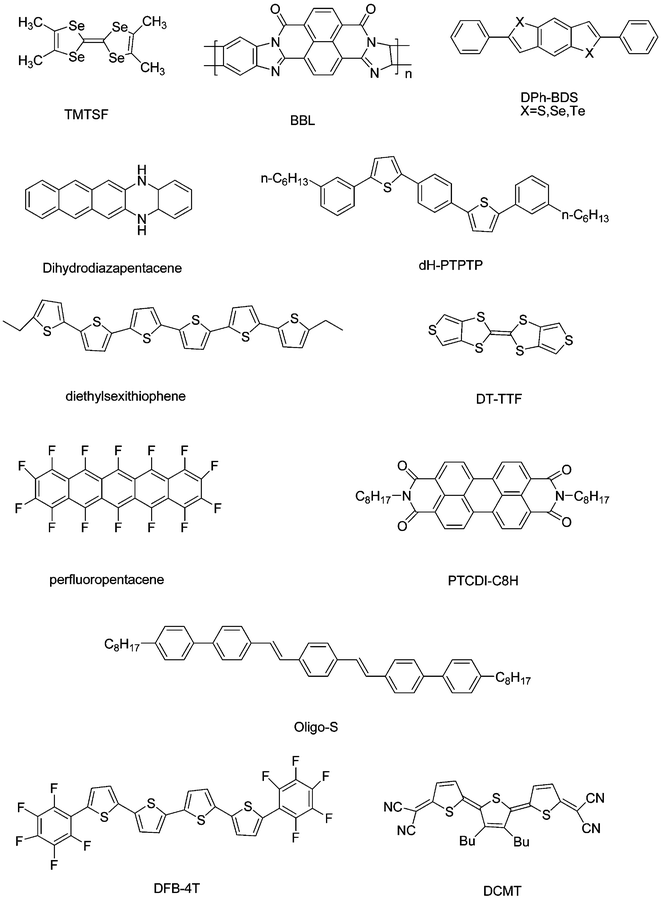 | ||
| Fig. 7 Several high-performance organic semiconductors which have been reported recently. | ||
| Compound | Type | Mobility/cm2 V−1 s−1 | Ion/Ioff | Reference |
|---|---|---|---|---|
| Poly(3-hexylthiophene) | p | 0.1 | >106 | 47 |
| α,ω-Dihexyl-sexithiophene | p | 0.13 | >104 | 43 |
| F16-CuPc | n | 0.03 | 5 × 104 | 61 |
| DCMT | n | 0.2 | >106 | 70 |
| TMTSF (single crystal) | p | 0.2 | NR | 88 |
| Rubrene (single crystal) | p | 15.4 | NR | 19 |
| Pentacene (single crystal) | p | 0.3 | >105 | 92 |
| Oligo-S | p | 0.12 | >106 | 89 |
| DT-TTF | p | 1.4 | NR | 91 |
| DFB-4T | n | 0.08 | >105 | 61 |
| Dihydrodiazapentacene | p | 0.006 | 5 × 103 | 57 |
| dH-PTPTP | p | 0.054 | 4 × 104 | 90 |
| BBL | n | 0.1 | 2 × 103 | 73 |
| DPh-BDS | p | 0.17 | 105 | 58 |
| PTCDI-C8H | n | 0.6 | >105 | 69 |
| Phthalocyanine | p | 0.11 | 105 | 54 |
| Pentacene | p | 1.5 | 108 | 27 |
| DHF-6T | n | 0.02 | 105 | 63 |
| α,α′-Diethylsexithiophene | p | 1.1 | 104 | 45 |
| α-Sexithiophene | p | 0.03 | >106 | 41 |
| Dibenzothienobisbenzodithiophene | p | 0.2 | >106 | 55 |
| Bisdithienothiophene | p | 0.05 | 108 | 56 |
| Perfluoropentacene | n | 0.11 | 105 | 65 |
6.2 Organic single crystal FETs
In the last three years, much work has been devoted to single crystal FETs. The highest charge mobility of 15.4 cm2 V−1 s−1 was reported for OFETs based on rubrene single crystals recently. The highest mobility is found in single crystals mainly due to molecular ordering that permits good overlapping of the π–π orbitals. Compared to single crystals, organic thin films are often associated with grain boundaries and interfacial disorder. Indeed, currently these structural defects are the major factor which limits the performance in devices fabricated on single crystals of organic semiconductors, so investigations on single crystals are necessary and there has been significant recent progress in this area.There are two methods often used in organic single crystal FETs: one way is that the single crystal is transferred to the electrodes which were made beforehand, another way is that the insulator is deposited onto the single crystal. Because crystals are very thin and rigid, techniques used in organic single crystal FETs are improved.
Recently, Wang and co-workers reported on the fabrication of OFETs on single pentacene microcrystals grown directly on a polymer film. The mobility was 1.2 cm2 V−1 s−1 for multilayer crystals of pentacene.93 The single microcrystals could be grown as large as 100 µm by controlling the substrate temperature. This work provides a route via which the grown technique may be applicable to the growth of single crystals.
Organic single crystal FETs were reported by Butko et al. The OFETs exhibited a hole mobility up to 0.3 cm2 V−1 s−1 and on/off ratios more than 105.92 The crystals were grown by horizontal physical vapor transport in a stream of ultrahigh purity argon. Perylene was used as insulator which was deposited on the top of the crystal. The large density of traps introduced in the process of OFET fabrication may affect the device performance.
Torrent fabricated organic single crystal field-effect transistors based on single crystals of the organic semiconductor dithiophene-tetrathiafulvalene (DT-TTF).91 A very simple and fast method was used to form the crystals: a warm saturated solution of DT-TTF in chlorobenzene was poured over the electrodes, and when the solvent evaporated at room temperature, long and thin crystals formed, some of which connected two of the microfabricated gold electrodes by van der Waal's forces. Using the drop casting method, a charge mobility of 1.4 cm2 V−1 s−1 can be obtained. But this fabrication method is only applicable to the characteristics of crystals similar to that of DT-TTF.
Sundar and coworkers have recently introduced a method to fabricate high-performance OFETs on the surface of freestanding organic rubrene crystals.19 The transistors were constructed by laminating a monolithic elastomeric transistor stamp against the surface of a crystal. The important advantages, compared with the Si-based technique, of the elastomeric technique are that the elastomeric stamp can be compatible with thicker substrates, rigid crystals and the technique is non-destructive and reversible. They explored the dependence of the mobility on the orientation of the transistor channel relative to the crystallographic axes and first observed a strong anisotropy of the field-effect mobility within the a–b plane of a single crystal of rubrene. The mobilities measured along the b and a axes of a rubrene single crystal are 15.4 cm2 V−1 s−1 and 4.4 cm2 V−1 s−1, respectively, which may be a result of stronger overlap of the electronic π orbitals along the b axis.
6.3 Organic light-emitting field-effect transistors (O-LEFTs)
Recently, Hepp et al. first reported O-LEFTs based on tetracene.94 As we know, organic light-emitting diodes (OLEDs) are operated in ambipolar operation mode which may lead to carrier recombination, formation of excitons, and light emission. However, OFETs are typically operated in unipolar accumulation mode. So OFETs are incapable of forming excitons and light emission by themselves. The emergence of O-LEFTs is a new development of OFETs in truth recently.The light emission zone was only found near the drain electrode and no shift was observed with varying gate voltages. They explained the phenomena using imperfections theory: bad contacts between electrodes were formed due to the different etching processes. The imperfections held back the transfer of holes to the drain electrode and a strong electric field generated between the drain electrode and the hole accumulation layer at the gate oxide. Under those conditions, electron injection may be possible.
O-LEFTs produced by polymers such as poly[9,9-di(ethylhexyl)fluorene] and poly[2-methoxy-5-(2′-ethylhexyloxy)-1,4-phenylenevinylene] (MEH-PPV) have also been reported.95,96 Sakanoue and coworkers have prepared visible O-LEFTs based on MEH-PPV with bottom-contact electrodes.96 Orange light emission was observed when the devices with Cr/Au or Al/Au as the electrodes were operated in vacuum and the light intensity could be controlled by the gate voltage. When a negative bias voltage was applied to the gate, holes were induced at the interface between MEH-PPV and SiO2. The holes were injected from the Au electrode, and at high drain voltages electrons were also injected into MEH-PPV from the Cr or Au electrodes, which resulted in carrier recombination. Light emission could not be observed for the devices without Au electrodes, indicating that the Au layers were essential for injection of holes.
Currently, although the mechanism of O-LEFTs is not very clear, undoubtedly, O-LEFTs would constitute a crucial building block for applications in optical information technology and nanotechnology.
7. Prospects and problems of OFETs that exist
There has been tremendous progress in OFET performance since it was first described in 1987. Currently, OFETs can rival the performance of FETs based on amorphous silicon (a-Si : H). Organic materials hold great potential for realizing low-cost flexible electronic devices and have become one of the most important components of organic electronics. At present, a large scale organic complementary integrated circuit has been reported to apply to commerce. However, there are a lot of questions for us to solve. The challenge includes the performance of organic semiconductors, the device stability and the fabrication techniques etc. If organics are to move up to large scale applications in the future, these questions must be resolved well.First, we talk about the performance of organic semiconductors. Currently, high performances are obtained for OFETs based on only pentacene and fused oligomers. The mobility can be about 1 cm2 V−1 s−1, ION/IOFF > 108. Compared to a-Si devices, the mobility is low. If the mobilities of organic semiconductors can achieve values near 10 cm2 V−1 s−1, the competitive landscape for applications would be very optimistic. Recent results for transistors based on rubrene single crystals show the mobility at room temperature can be higher than 10 cm2 V−1 s−1. These high mobilities are only found in single crystal FETs. Most transistors are based on polycrystalline films of organic semiconductors. The grain boundaries would form many traps, which hold back the transfer of carriers. Achieving that high mobility, we will be curious to determine whether the performance limit of organic semiconductors has already been reached with current materials.
The structure–property issues in organic semiconductors are still unclear and need to be addressed in future work. Understanding the transport mechanism in OFETs can help us to design high performance materials. Moreover, the series of organic materials are limited, especially for n-type semiconductors. Thus, to fabricate and synthesize a lot of stable organic semiconductors with high performance would be very important, which is a challenge for materials chemists. Dielectric materials are extremely crucial for OFETs. In general, organic dielectric materials have relatively low dielectric constants compared with inorganic materials. High-performance dielectric materials would be very important with the development of organic electronic devices.
Second, we mention the fabrication techniques for OFETs. Substantial improvements have taken place in OFET fabrication techniques, especially for solution-processed techniques, which are low cost compared to vacuum evaporation techniques because the former eliminate the vacuum conditions and make the fabrication process simple. We consider that OFETs possessing the advantages of low cost techniques, such as spin-coating and printing, have a bright application future. Many organic semiconductor molecules such as pentacene and sexithiophene have very poor solubility and they are not amenable to solution-processed techniques. Considering that, polymers are the most promising candidates due to their good solubility. However, exploring the techniques, the mobilities are lower by one order of magnitude than those of vacuum deposited devices, so the solution-processed techniques need further improvement.
Finally, the factors affecting device performance are also paid attention. As we mentioned above, during the fabrication process, every step can form traps, which degenerate the device performance. Therefore, fundamental issues related to device degradation and interfacial interactions are becoming important guidelines to OFET improvements. The effects coming from the purity of the materials or from the environment should be minimized.
All in all, there is a long way to go for OFETs in spite of their excellent performance achieved in recent years. Good stability and long lifetimes of devices with low cost are required in order to fully realize the benefits of organic electronics.
Acknowledgements
The authors gratefully acknowledge financial support from the National Natural Science Foundation of China (NNSFC), the Major State Basic Research Development Program and Chinese Academy of Sciences.References
- H. Koezuka, A. Tsumura and T. Ando, Synth. Met., 1987, 18, 699 CrossRef CAS.
- C. D. Dimitrakopoulos and P. R. L. Malenfant, Adv. Mater., 2002, 14, 99 CrossRef CAS.
- G. Horowitz, Adv. Mater., 1998, 10, 365 CrossRef CAS.
- G. Horowitz, J. Mater. Chem., 1999, 9, 2021 RSC.
- M. Pope and C. E. Swenberg, Electronic Processes, in Organic Crystals and Polymers, Oxford University Press, Oxford, 1999 Search PubMed.
- C. D. Sheraw, L. Zhou, J. R. Huang, D. J. Gundlach, T. N. Jackson, M. G. Kane, I. G. Hill, M. S. Hammond, J. Campi, B. K. Greening, J. Francl and J. West, Appl. Phys. Lett., 2002, 80, 1088 CrossRef CAS.
- B. Comiskey, J. D. Albert, H. Yoshizawa and J. Jacobson, Nature, 1998, 394, 253 CrossRef CAS.
- B. K. Crone, A. Dodabalapur, A. Gelperin, L. Torsi, H. E. Katz, A. J. Ovinger and Z. Bao, Appl. Phys. Lett., 2001, 78, 2229 CrossRef CAS.
- C. J. Drury, C. M. J. Mutsaers, C. M. Hart, M. Matters and D. M. de Leeuw, Appl. Phys. Lett., 1998, 73, 108 CrossRef CAS.
- G. H. Gelinck, T. C. T. Geuns and D. de Leeuw, Appl. Phys. Lett., 2000, 77, 1487 CrossRef CAS.
- P. Mach, S. J. Rodriguez, R. Nortrup, P. Wiltzius and J. A. Rogers, Appl. Phys. Lett., 2001, 78, 3592 CrossRef CAS.
- H. E. A. Huitema, G. H. Gelinck, J. B. P. H. van der Putten, K. E. Kuijk, C. Hart, E. Cantatore, P. T. Herwigm, A. J. J. M. van Breemen and D. M. Leeuw, Nature, 2001, 414, 599 CrossRef CAS.
- A. Dodabalapur, J. Laquindanum, H. E. Katz and Z. Bao, Appl. Phys. Lett., 1996, 69, 4227 CrossRef CAS.
- B. K. Crone, A. Dodabalapur, R. Sarpeshkar, R. W. Filas, Y. Y. Lin, Z. Bao, J. H. O'Neill, W. Li and H. E. Katz, J. Appl. Phys., 2001, 89, 5125 CrossRef CAS.
- Y. Y. Lin, A. Dodabalapur, R. Sarpeshkar, Z. Bao, W. Li, K. Baldwin, V. R. Raju and H. E. Katz, Appl. Phys. Lett., 1999, 74, 2714 CrossRef CAS.
- C. D. Sheraw, J. A. Nichols, D. J. Gundlach, J. R. Huang, C. C. Kuo, H. Klauk, T. N. Jackson, M. G. Kane, J. Campi, F. P. Cuomo and B. K. Greening, Tech. Dig. Int. Electron Devices Meet., 2000, 619 Search PubMed.
- B. K. Crone, A. Dodabalapur, Y. Y. Lin, R. W. Filas, Z. Bao, A. Laduca, R. Sarpeshkar, H. E. Katz and W. Li, Nature, 2000, 403, 521 CrossRef CAS.
- Y. Taur and T. H. Ning, Fundamentals of Modern VLSI Devices, Cambridge University Press, Cambridge, 1998, p. 11 Search PubMed.
- V. C. Sundar, J. Zaumseil, V. Podzorov, E. Menard, R. L. Willett, T. Someya, M. E. Gershenson and J. A. Rogers, Science, 2004, 303, 1644 CrossRef CAS.
- A. J. Heeger, S. Kivelson, J. R. Schrieffer and W. P. Su, Mod. Phys., 1988, 60, 781 Search PubMed.
- S. F. Nelson, Y.-Y. Lin, D. J. Gundlach and T. N. Jackson, Appl. Phys. Lett., 1998, 72, 1854 CrossRef CAS.
- G. Horowitz and P. Delannoy, J. Appl. Phys., 1991, 70, 469 CrossRef CAS.
- G. Horowitz, R. Hajlaoui and P. Delannoy, J. Phys. III (Paris), 1995, 5, 355 Search PubMed.
- P. G. Le Comber and W. E. Spear, Phys. Rev. Lett., 1970, 25, 509 CrossRef CAS.
- D. M. de Leeuw, M. M. J. Simenon, A. R. Brown and R. E. F. Einerhand, Synth. Met., 1997, 87, 53 CrossRef.
- D. J. Gundlach, Y. Y. Lin, T. N. Jackson, S. F. Nelson and D. G. Schlom, IEEE Electron Device Lett., 1997, 18, 87 CrossRef CAS.
- Y. Y. Lin, D. J. Gundlach, S. F. Nelson and T. N. Jackson, IEEE Electron Device Lett., 1997, 18, 606 CrossRef CAS.
- H. Klauk, M. Halik, U. Zschieschang, G. Schmid, W. Radlik and W. J. Weber, J. Appl. Phys., 2002, 92, 5259 CrossRef CAS.
- A. R. Brown, A. Pomp, D. M. de Leeuw, D. B. M. Klaassen, E. E. Havinga, P. Herwig and K. Müllen, J. Appl. Phys., 1996, 79, 2136 CrossRef CAS.
- P. T. Herwig and K. Müllen, Adv. Mater., 1999, 11, 480 CrossRef.
- A. Afzali, C. D. Dimitrakopoulos and T. L. Breen, J. Am. Chem. Soc., 2002, 124, 8812 CrossRef CAS.
- A. Afzali, C. D. Dimitrakopoulos and T. O. Graham, Adv. Mater., 2003, 15, 2066 CrossRef CAS.
- C. D. Sheraw, T. N. Jackson, D. L. Eaton and J. E. Anthony, Adv. Mater., 2003, 15, 2009 CrossRef CAS.
- H. Meng, M. Bendikov, G. Mitchell, R. Helgeson, F. Wudl, Z. Bao, T. Siegrist, C. Kloc and C. H. Chen, Adv. Mater., 2003, 15, 1090 CrossRef CAS.
- S. Hotta and K. Waragai, J. Mater. Chem., 1991, 1, 835 RSC.
- F. Garnier, A. Yassa, R. Hajlaoui, G. Horowitz, F. Deloffre, B. Servet, S. Ries and P. Alnot, J. Am. Chem. Soc., 1993, 115, 8716 CrossRef CAS.
- G. Horowitz, D. Fichou, X. Z. Peng, Z. G. Xu and F. Garnier, Solid State Commun., 1989, 72, 381 CrossRef CAS.
- F. Garnier, Pure Appl. Chem., 1996, 68, 1455 CrossRef CAS.
- H. E. Katz, J. Mater. Chem., 1997, 7, 369 RSC.
- F. Garnier, G. Horowitz, X. Z. Peng and D. Fichou, Synth. Met., 1991, 45, 163 CrossRef CAS.
- A. Dodabalapur, L. Torsi and H. E. Katz, Science, 1995, 268, 270 CrossRef CAS.
- H. E. Katz, L. Torsi and A. Dodabalapur, Chem. Mater., 1995, 7, 2235 CrossRef CAS.
- C. D. Dimitrakopoulos, B. K. Furman, T. Graham, S. Hedge and S. Purushothaman, Synth. Met., 1998, 92, 47 CrossRef CAS.
- G. Horowitz and M. E. Hajlaoui, Adv. Mater., 2000, 12, 1046 CrossRef CAS.
- M. Halik, H. Klauk, U. Zschieschang, G. Schmid, S. Ponomarenko, S. Kirchmeyer and W. Weber, Adv. Mater., 2003, 15, 917 CrossRef CAS.
- Z. Bao, A. Dodabalapur and A. J. Lovinger, Appl. Phys. Lett., 1996, 69, 4108 CrossRef CAS.
- H. Sirringhaus, N. Tessler and R. H. Friend, Science, 1998, 280, 1741 CrossRef CAS.
- A. R. Brown, D. M. de Leeuw, E. E. Havingga and A. Pomp, Synth. Met., 1994, 68, 65 CrossRef CAS.
- A. R. Brown, C. P. Jarrett, D. M. de Leeuw and M. Matters, Synth. Met., 1997, 88, 37 CrossRef CAS.
- Z. Bao, A. J. Lovinger and A. Dodabalapur, Appl. Phys. Lett., 1996, 69, 3066 CrossRef CAS.
- Z. Bao, A. J. Lovinger and A. Dodabalapur, Adv. Mater., 1997, 9, 42 CAS.
- R. Rella, A. Serra, P. Siciliano, A. Tepore and A. Zoeco, Langmuir, 1997, 13, 6562 CrossRef CAS.
- K. Y. Law, Chem. Rev., 1993, 93, 449 CrossRef CAS.
- J. Zhang, J. Wang, H. Wang and D. Yan, Appl. Phys. Lett., 2004, 84, 3066.
- H. Sirringhaus, R. H. Friend, C. Wang, J. Leuninger and K. Müllen, J. Mater. Chem., 1999, 9, 2095 RSC.
- X. C. Li, H. Sirringhaus, F. Garnier, A. B. Holmes, S. C. Moratti, N. Feeder, W. Clegg, S. J. Teat and R. H. Friend, J. Am. Chem. Soc., 1998, 120, 2206 CrossRef CAS.
- Q. Miao, T. Q. Nguyen, T. Someya, G. B. blanchet and C. Nuckolls, J. Am. Chem. Soc., 2003, 125, 10284 CrossRef CAS.
- K. Takimiya, Y. Kunugi, Y. Konda, N. Niihara and T. Otsubo, J. Am. Chem. Soc., 2004, 126, 5084 CrossRef CAS.
- C. R. Newman, C. D. Frisbie, D. A. da S. Filho, J. L. Bredas, P. C. Ewbank and K. R. Mann, Chem. Mater., 2004 DOI:10.1021/cm049391x (ASAP Article).
- H. E. Katz and Z. Bao, J. Phys. Chem. B, 2000, 104, 671 CrossRef CAS.
- Z. Bao, A. J. Lovinger and J. Brown, J. Am. Chem. Soc., 1998, 120, 207 CrossRef CAS.
- A. Facchetti, M. H. Yoon, C. L. Stern, H. E. Katz and T. J. Marks, Angew. Chem., Int. Ed., 2003, 42, 3900 CrossRef CAS.
- A. Facchetti, Y. Deng, A. Wang, Y. Koide, H. Sirringhaus, T. J. Marks and R. H. Friend, Angew. Chem., Int. Ed., 2000, 39, 4547 CrossRef CAS.
- S. B. Heidenhain, Y. Sakamoto, T. Suzuki, A. Miura, H. Fujikawa, T. Mori, S. Tokito and Y. Taga, J. Am. Chem. Soc., 2000, 122, 10240 CrossRef CAS.
- Y. Sakamoto, T. Suzuki, M. Kobayashi, Y. Gao, Y. Fukai, Y. Inoue, F. Sato and S. Tokio, J. Am. Chem. Soc., 2004, 126, 8138 CrossRef CAS.
- J. R. Ostrick, A. Dodabalapur, L. Torsi, A. J. Lovinger, E. W. Kwock, T. M. Tiller, M. Galvin, M. Berggren and H. E. Katz, J. Appl. Phys., 1997, 81, 6804 CrossRef CAS.
- G. Horowitz, F. Kouki, P. Spearman, D. Fichou, C. Nogues, X. Pan and F. Garnier, Adv. Mater., 1996, 8, 242 CAS.
- D. Y. Zhang, F. So and S. R. Forrest, Appl. Phys. Lett., 1991, 59, 823 CrossRef.
- P. R. L. Malenfant, C. D. Dimitrakopoulos, J. D. Gelorme, L. L. Kosbar, T. O. Graham, A. Curioni and W. Andreoni, Appl. Phys. Lett., 2002, 80, 2517 CrossRef CAS.
- R. J. Chesterfield, C. R. Newman, T. M. Pappenfus, P. C. Ewbank, M. H. Haukaas, K. R. Mann, L. L. Miller and C. D. Frisbie, Adv. Mater., 2003, 15, 1278 CrossRef CAS.
- T. M. Pappenfus, R. J. Chesterfield, C. D. Frisbie, K. R. Mann, J. Casado, J. D. Raff and L. L. Miller, J. Am. Chem. Soc., 2002, 124, 4184 CrossRef CAS.
- A. Dodabalapur, H. E. Katz, L. Torsi and R. C. Haddon, Appl. Phys. Lett., 1996, 68, 1108 CrossRef CAS.
- A. Babel and S. A. Jenekhe, J. Am. Chem. Soc., 2003, 125, 13656 CrossRef CAS.
- O. D. Jurchescu, J. Baas and T. T. M. Palstra, Appl. Phys. Lett., 2004, 84, 3061 CrossRef CAS.
- C. D. Dimitrakopoulos, A. R. Brown and A. Pomp, J. Appl. Phys., 1996, 80, 2501 CrossRef CAS.
- C. D. Dimitrakopoulos and D. J. Mascaro, IBM J. Res. Dev., 2001, 45, 11 CrossRef CAS.
- K. Xiao, Y. Q. Liu, G. Yui and D. B. Zhu, Appl. Phys. A, 2003, 77, 367 CrossRef CAS.
- Langmuir–Blodgett Films, ed. G. Roberts, Plenum Press, New York, 1990 Search PubMed.
- A. Kumar and G. M. Whitesides, Appl. Phys. Lett., 1993, 63, 2002 CrossRef.
- Y. Xia, E. Kim, X. M. Zhao, J. A. Rogers, M. Prentiss and G. M. Whitesides, Science, 1996, 273, 347 CrossRef CAS.
- X. M. Zhao, Y. Xia and G. M. Whitesides, Adv. Mater., 1996, 8, 837 CrossRef CAS.
- E. Kim, Y. Xia and G. M. Whitesides, Nature, 1995, 376, 581 CrossRef CAS.
- E. Kim, Y. Xia, X. M. Zhao and G. M. Whitesides, Adv. Mater., 1997, 9, 651 CAS.
- Y. Xia and G. M. Whitesides, Angew. Chem., Int. Ed., 1998, 37, 550 CrossRef CAS.
- N. L. Jeon, K. Finnie, K. Branshaw and R. G. Nuzzo, Langmuir, 1997, 13, 3382 CrossRef CAS.
- Y. Xia and G. M. Whitesides, J. Am. Chem. Soc., 1995, 117, 3274 CrossRef CAS.
- Z. Wang, J. Yuan, J. Zhang, R. Xing, D. Yan and Y. Han, Adv. Mater., 2003, 12, 1009 CrossRef.
- M. S. Nam, A. Ardavan, R. J. Cava and P. M. Chaikin, Appl. Phys. Lett., 2003, 83, 4782 CrossRef CAS.
- T. C. Gorjanc, I. Levesque and M. D'lorio, Appl. Phys. Lett., 2004, 84, 930 CrossRef CAS.
- M. Mushrush, A. Facchetti, M. Lefenfeld, H. E. Katz and T. J. Marks, J. Am. Chem. Soc., 2003, 125, 9414 CrossRef CAS.
- M. M. Torrent, M. Durkut, P. Hadley, X. Ribas and C. Rovira, J. Am. Chem. Soc., 2004, 126, 984 CrossRef.
- V. Y. Butko, X. Chi, D. V. Lang and A. P. Ramirez, Appl. Phys. Lett., 2003, 83, 4773 CrossRef CAS.
- G. Wang, Y. Luo and P. H. Beton, Appl. Phys. Lett., 2003, 83, 3108 CrossRef CAS.
- A. Hepp, H. Heil, W. weise, M. Ahles, R. Schmechel and H. von Seggern, Phys. Rev. Lett., 2003, 91, 157406 CrossRef.
- M. Ahles, A. Hepp, R. Schmechel and H. von Seggern, Appl. Phys. Lett., 2004, 84, 428 CrossRef CAS.
- T. Sakanoue, E. Fujiwara, R. Yamada and H. Tada, Appl. Phys. Lett., 2004, 84, 3037 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2005 |
