Research Highlights
First published on 26th November 2007
Molecular logic gates based on fluorescent chemosensors
The construction of molecular gates with the potential for calculation on the nanometre scale is an intriguing challenge. In earlier work, logic gates have been developed by means of a microfluidic device using surface tension-based passive pumping and fluidic resistance.1 Many molecular logic gates that are able to perform logic operations (AND, OR, XOR, NAND, half-adder, etc.) employ fluorescence changes upon inputs such as pH, metal ions and anions. On the basis of such fluorescent chemosensors, Songzi Kou et al. have designed a molecular logic gate that is implemented in a programmable microfluidic device.2 In analogy with electronic computers, the microfluidic channels are the wires that distribute the information, while the reaction chambers are the logic operators. The device consists of two layers, one of which includes a circulation loop that communicates with five pairs of inlet and outlet channels. The other (bottom) layer consists of ten microchannels and five sets of individual microvalves and twin valves that are operated pneumatically by controlling the flow of nitrogen gas into the valves. By actuation of the valves, the solutions in the circulation loop are pumped round, thus resulting in a mixing process. The device is loaded first with the fluorescent chemosensor solution, and afterwards with special pH-conditioning solutions or metal-ion solutions (the “input”). The “output” is the fluorescence intensity of the chemosensors. These fluorescent chemosensors, fluorescein or rhodamine B derivatives, have a strong green and red fluorescence emission, respectively, but can be quenched at certain pH. Based on these fluorescent chemosensors, a half-adder molecular logic gate is composed of an XOR (eXclusive OR) gate and an AND gate in a combinatorial circuit (Fig. 1). Furthermore, INHIBIT logic gates are created using the quenching effect of Cu2+ ions on a Cu2+-selective fluorescein derivative. The quenching of Cu2+ ions can be inhibited by addition of a copper-binding protein (transferrin), which is also demonstrated. The low sample consumption, high throughput and potential automation of molecular logic gates realized in a microdevice could be an extremely versatile approach for the construction of molecular computing systems.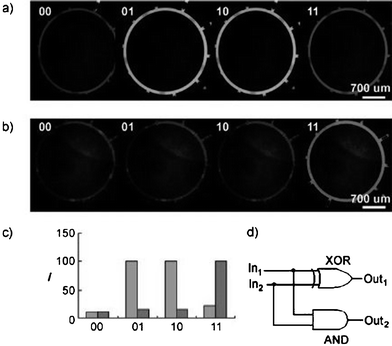 | ||
| Fig. 1 Logic gates realized in a microfluidic chip on the basis of fluorescent chemosensors. Fluorescence images in the presence of two H+ inputs for (a) an exclusive OR gate (XOR) and (b) an AND gate. (a) The output exhibits an intensive fluorescence signal, in the case where one of the input channels contains H+, while in the AND molecular gate, the fluorescence intensity reaches a maximum only if both input channels supply H+ at the same time. The fluorescence intensities for each output are shown in (c). A half-adder circuit is formed by an XOR and an AND gate as schematically shown in (d). (Reprinted with permission from Kou et al.2 Copyright 2007 VCH-Wiley). | ||
Food- and odour-evoked behaviour in worms
Neural circuits are sensors that receive signals from the environment, determine the location and temporal course of this sensory input, and regulate behaviour on the basis of this information. Sensory inputs can lead to long-lasting and complex behavioural sequences. One example is the chemotaxis of animals to food odours or tastes, during which animals reach an attractant source by regulating turns over many minutes. An essential link from the sensory input to behaviour is the communication between different neuronal circuits.In a recent article, researchers from The Rockefeller University (New York) and Stanford University addressed the question of how odours can affect neuron activity, and studied the functional connectivity between different neurons that initiates the transformation of chemosensory inputs into behaviour.3 The animal that is investigated is the nematode Caenorhabditis elegans (C. elegans), which is a well-investigated model organism containing just 302 neurons with known synaptic connections. It is known that a pair of olfactory neurons (so-called AWC) is important for the food-seeking behaviour of C. elegans, more precisely, AWC neurons direct chemotaxis to odours. During the experiment, the worm is trapped in a narrow channel of a microfluidic device in such a way that the worm nose reaches another wide channel that contains buffer solutions or odour stimuli (Fig. 2). The odour response of olfactory AWC neurons is monitored by using a calcium imaging assay, in which the change of fluorescence intensity of a calcium binding sensor is monitored. The response of AWC neurons of individual animals is observed for altering conditions (buffer, isoamyl alcohol odour, bacterial-conditioned medium), and the response in fluorescence intensity can be seen within seconds. The findings demonstrate that AWC neurons have basal activity in the absence of odour, are inhibited by odour and are stimulated by odour removal. It has been found in former studies that AWC neurons synapse onto several interneurons (called AIB and AIY), which have an additional effect on odour-evoked behaviour. In this current study, the researchers show that AWC neurons activate the AIB interneurons through glutamate receptors, while the AWC neurons inhibits AIY interneurons through glutamate-gated chloride channels.
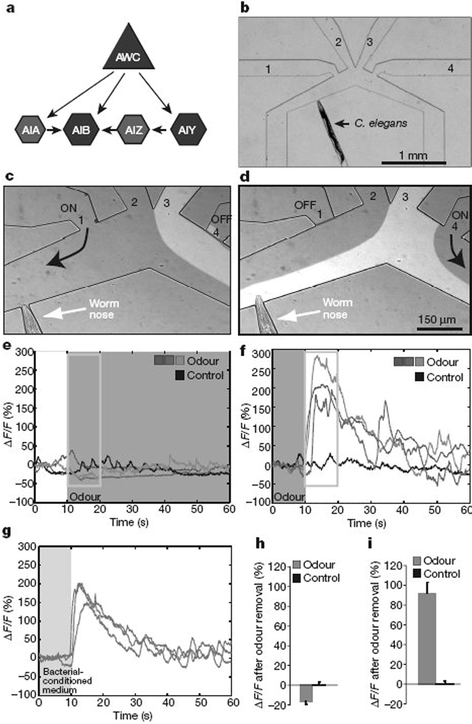 | ||
| Fig. 2 Response of olfactory neurons called AWC and several interneurons (AIB, AIY,…see (a)) in the worm C. elegans by stimulation with odours. The worm is trapped in a microfluidic chip and exposed to buffer or odours (b–d). The response of the neurons is recorded by means of a calcium sensor. In this assay, the fluorescence intensity increases upon neural activation, which is observed after removal of odour-containing solutions (e–i). (Reprinted with permission from Macmillan Publishers Ltd: Nature, Chalasani et al.,3 copyright 2007). | ||
The presented methods facilitate the investigation of food-seeking behaviour of C. elegans on a circuit level. In further studies it might be possible to find explanations for stochastic behaviours, the generation of persistent behavioural responses to transient stimuli, and temporal integration of the nervous system.
Microfluidic cell culture system
During the last decade, the use of microfluidic platforms for cell analytical applications has extensively increased. While the implementation of microsystems technology for short term cell treatment and manipulation has become common standard, the implementation of microchips for long-term studies over days or weeks is still challenging. However, there is a great demand for such systems driven by the emerging research in stem cell biology and systems biology. For these applications it is particularly important to culture cells and maintain cell viability over a long period of time in individual chambers while the culture conditions of each chamber can be controlled precisely. The development of such a cell culture screening system has been recently presented by Stephen R. Quake and co-workers.4 The fully automated system is based on a microfluidic chip with 96 addressable culture chambers, each having a volume of 60 nL (Fig. 3). Each chamber is individually addressable allowing one to customize distinct culture conditions from chamber to chamber and as a function of time, i.e. parameters such as cell seeding density, composition of culture medium and feeding schedule could be regulated. Different mixtures of reagents can be supplied through 16 different inputs, and a mixing section is used to individually formulate the composition of reagents fed to each chamber. Likewise, fixing and staining reagents can be supplied at the end of the experiments. The cell seeding density can be adjusted by using a sequential loading procedure, during which different cell samples can be supplied into the culture chambers. It is furthermore possible to vary the surface treatments for each chamber by using different protein adhesion coatings before starting the experiments. During the entire course of the experiments, growth medium is continuously refreshed in each cell culture chamber. The authors demonstrate that the microfluidic platform is capable of sustaining proliferation of human primary mesenchymal stem cells. Moreover, osteogenic differentiation is induced and the influence of transient stimulation is studied quantitatively. The duration of stimulation is varied from 0 to 168 h. The results suggest that the human primary mesenchymal stem cells need a minimum of 4 days of stimulation to fully commit to differentiation into the osteogenic lineage. Furthermore, the evolution of cell motility within 9 days for stimulated and non-stimulated cells is characterised. Not stimulated cells have a constant mobility over the length of the experiment, while cells that are exposed to osteogenic differentiation medium exhibit a continuous decrease. The effect of cell differentiation factors on mobility is reversible in the case where the stimulation time was below 96 h, i.e. cell mobility reaches normal mobility after removal of stimulating factors.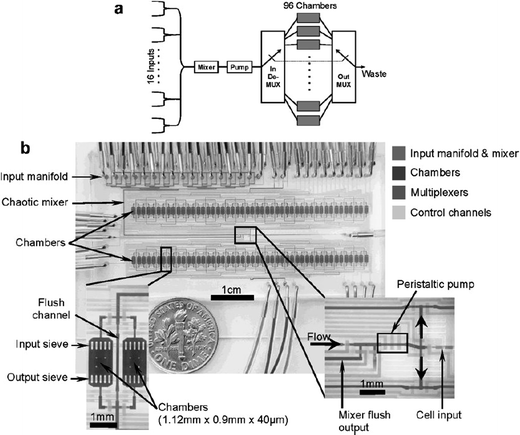 | ||
| Fig. 3 Chip design for automated long-term cell culture. Cells cultured in one of the 96 chambers maintain cell viability for weeks. The chip is designed for quantitative studies of proliferation and osteogenic differentiation of human primary mesenchymal stem cells. (Reprinted from Gómez-Sjöberg et al.4 Copyright 2007 American Chemical Society). | ||
Continuous retrovirus production
Retroviruses contain an RNA genome and replicate via a DNA intermediate that can be integrated in the genome of the host cell. The viruses are highly infectious for eukaryotic cells and since the genome of retroviruses mutates often, the viruses can grow resistant to antiviral pharmaceuticals very quickly. On the other hand, retroviruses are of clinical significance, and are commonly used vectors in gene therapy trials. Due to their short half-life time of a few hours, however, the use of the viruses in clinical applications is limited. Rapid production and purification of retroviruses would significantly enhance their applicability. In the current issue of Lab on a Chip, Martin L. Yarmush and co-workers describe how retrovirus production can be improved by use of a novel microfluidic device.5 The transparent silicon elastomer device consists of 8 parallel channels and various channel heights (50, 100, 150 µm). The growth of a retroviral packaging cell line is investigated in the microfluidic devices for several days. The results are compared to the growth kinetics under static conditions in a 6-well culture plate, and are found to be almost equal. The production rates of the virus are determined for continuous periods of 24 hours at 37 °C. Again, static and microfluidic conditions show no significance difference in the virus production per cell and per day. However, the use of the microfluidic device facilitates precise control over the flow streams and allows integration of a downstream cold storage area. As the retrovirus is known to degrade rapidly at 37 °C, the immediate cold storage could prevent virus degradation. Indeed, the researchers could demonstrate the increase of virus production level up to a factor of 3.7. The beauty of the microfluidic concept is that cellular transduction can be performed on the same chip by integration of an additional area, in which target cells are cultured for immediate infection.Petra S. Dittrich
ISAS, Dortmund, Germany
dittrich@isas.de
References
- M. W. Toepke, V. V. Abhyankar and D. J. Beebe, Microfluidic logic gates and timers, Lab Chip, 2007, 7, 1449–1453 RSC.
- S. Kou, H. N. Lee, D. Van Noort, K. M. K. Swammy, S. H. Kim, J. H. Soh, K.-M. Lee, S.-W. Nam, J. Yoon and S. Park, Fluorescent Molecular Logic Gates Using Microfluidic Devices, Angew. Chem., Int. Ed., 2007, 46 DOI:10.1002/anie.200703813.
- S. H. Chalasani, N. Chronis, M. Tsunozaki, J. M. Gray, D. Ramot, M. B. Goodman and C. I. Bergmann, Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans, Nature, 2007, 450, 63–70 CrossRef CAS.
- R. Gómez-Sjöberg, A. A. Leyrat, D. M. Pirone, C. S. Chen and S. R. Quake, Versatile, Fully Automated, Microfluidic Cell Culture System, Anal. Chem., 2007, 79, 8557–8563 CrossRef.
- H. N. Vu, Y. Li, M. Casali, D. Irimia, Z. Megeed and M. L. Yarmush, A microfluidic bioreactor for increased active retrovirus output, Lab Chip, 8 10.1039/b711577f.
| This journal is © The Royal Society of Chemistry 2008 |
