Self-assembly of a novel metal–organic coordination cage (MOCC) based on a new flexible dicarboxylate ligand: synthesis, crystal structure and magnetic property†
Fangna
Dai
a,
Haiyan
He
a,
Aiping
Xie
a,
Guodong
Chu
a,
Daofeng
Sun
*a and
Yanxiong
Ke
*b
aKey Lab for Colloid and Interface Chemistry of Education Ministry, Department of Chemistry, Shandong University, Jinan, 250100, P. R. China. E-mail: dfsun@sdu.edu.cn; Fax: +86 53188364218; Tel: +86 53188364218
bEngineering Research Center of Pharmaceutical Process Chemistry, Ministry of Education, School of Pharmacy, East China University of Science and Technology, 130 Meilong Road, Shanghai, 200237, P. R. China. E-mail: key@ecust.edu.cn
First published on 4th November 2008
Abstract
The self-assembly of flexible bended dicarboxylate ligand, 2,2′-(2,4,6-trimethyl-1,3-phenylene)bis(methylene)-bis(sulfanediyl)-dibenzoic acid (H2L), with Mn(OAc)2·4H2O resulted in the formation of a novel metal–organic coordination cage with binuclear manganese SBU as the vertex, which represents the first metal–organic coordination cage constructed from a flexible dicarboxylate ligand and a binuclear SBU.
The rational design and synthesis of metal–organic supramolecular architectures such as discrete metal–organic coordination cages (MOCCs), nanoball, metallamacrocycles and multidimensional open frameworks have attracted much attention from chemists due to their interesting structural topologies and potential applications in catalysis, separation, gas storage, etc.1–3 Selecting a suitable organic ligand with certain features, such as flexibility, appropriate angles and versatile binding modes, is crucial to the construction of a discrete cage or an open framework. In the past decades, many discrete metal–organic coordination cages or polygons have been synthesized and documented. In particular, Yaghi et al4 and Zaworotko et al5 reported the design and synthesis of discrete cages or polygons by using 120° angular dicarboxylate ligands. Robson et al.6 prepared and structurally characterized a [Cu12(tapp)8] cube-like cage, which was derived from the self-assembly of tri-bidentate ligand 2,4,6-triazophenyl-1,3,5-trihydroxybenzene (H3tapp) and copper(II) ions. Raymond et al synthesized a series of polyhedral structures based on biscatecholate ligands and tested their catalytic properties.7 The metal–organic cages constructed from N-containing organic ligands have also been independently reported by Fujita,8 Stang,9 and Hong et al.10
Most of the organic ligands used in the construction of metal–organic coordination cages are limited to a rigid angular carboxylate and N-containing ligand as well as biscatecholate ligand with appropriate chelating ability. The metal–organic coordination cages constructed from a flexible carboxylate ligand are quite rare.11 Recently, we designed a new flexible bended dicarboxylate ligand, namely, 2,2′-(2,4,6-trimethyl-1,3-phenylene)bis(methylene)-bis(sulfanediyl)dibenzoic acid (H2L, scheme 1). The bended geometry with an appropriate angle of the ligand makes it suitable for the construction of a discrete metal–organic coordination cage. In this communication, we report a novel metal–organic coordination cage, [Mn4(H2O)4L4(dmf)4]·6dmf·H2O (MOCC-3) with a new binuclear manganese unit as the secondary building unit (SBU).
 | ||
| Scheme 1 | ||
The organic ligand was synthesized by a one-step reaction between 2,4-bis(bromomethyl)-1,3,5-trimethylbenzene and 2-mercaptobenzoic acid in dry methanol according to the literature.12
Slow evaporation‡of the reaction mixture of H2L and Mn(CH3COO)2·4H2O in dmf/ethanol/H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in the presence of pyridine resulted in the formation of block crystals of MOCC-3, which was structurally characterized by single-crystal X-ray diffraction.§ The formula of [Mn4(H2O)4L4(dmf)4]·6dmf·H2O (MOCC-3), was further confirmed by elemental analysis and TGA.
1) in the presence of pyridine resulted in the formation of block crystals of MOCC-3, which was structurally characterized by single-crystal X-ray diffraction.§ The formula of [Mn4(H2O)4L4(dmf)4]·6dmf·H2O (MOCC-3), was further confirmed by elemental analysis and TGA.
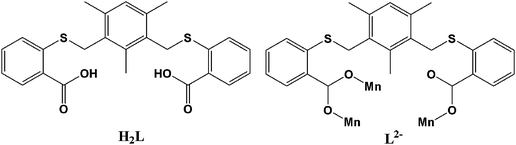
Single-crystal X-ray diffraction reveals that the crystal structure of MOCC-3 is formed by two kinds of slightly different discrete cages and crystallized in a monoclinic space groupP21/c. The asymmetric unit possesses four crystallographically independent manganese ions and four dicarboxylate ligands (ESI, Fig. S1 and S2).† Due to the similarity of the two cages formed by Mn1 + Mn2 and Mn3 + Mn4 units, only the cage generated by Mn1 + Mn2 unit is described below. Mn1 is coordinated by four carboxylate oxygen atoms from different L ligands, one terminal water molecule and one bridging water molecule in an octahedral geometry, while Mn2 is coordinated by two carboxylate oxygen atoms, one bridging water molecule and two dmf molecules in a triangle bipyramidal geometry, giving an average Mn–O distance of 2.140 Å. The two carboxylate groups of L ligand possess different coordination modes. One adopts a bidentate bridging mode to connect two manganese ions while the other one adopts a monodentate coordination mode to link one manganese ion (Scheme 1).
Mn1 and Mn2 are connected by two carboxylate groups, one bridging water molecule to form the binuclear SBU (Fig. 1a). Two such binuclear SBUs are further linked by four L ligands to result in the formation of a tetranuclear manganese metal–organic coordination cage (Fig. 1). The cage possesses C2 symmetry. The binuclear manganese units and the L ligands can be considered as the vertices and edges of the cage, respectively. The coordinated dmf molecules reside in the cage, which prevents other free solvent molecules from entering the cage. The L ligand is bent in the crystal structure with an average dihedral angle between the central benzene ring and the side benzene ring of 90.4° in an almost vertical arrangement, which results in the two carboxylate groups of L ligand in parallel arrangement to connect the manganese ions.
 | ||
| Fig. 1 (a) The binuclear SBU linked by two carboxylate groups and one coordinated water molecule, the carbon and nitrogen atoms of the dmf molecules in Mn2 ion are omitted for clarity; (b) schematic representation of the cage showing the structure of one of the four identical ligands that span the edges of the cage; (c) the cage crystal structure with coordinated dmf molecules inside; (d) space-fill representation of the cage with the coordinated dmf molecules in yellow color. | ||
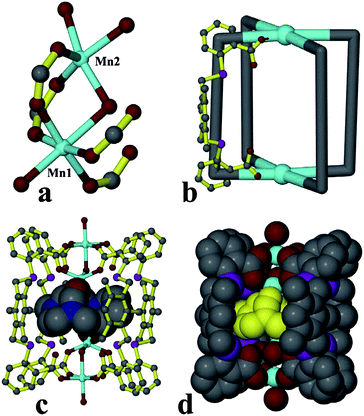
Although the two independent metal–organic coordination cages formed by {Mn1 + Mn2} and {Mn3 + Mn4} SBUs are uniform, the C–H⋯π interactions (3.787 Å) between the side benzene ring or the methylene group of L ligand in one cage and the central benzene ring of L ligand in an adjacent cage connect the discrete cages formed by {Mn1 + Mn2} and {Mn3 + Mn4} SBUs, respectively, to give rise to two different layers with different cavities (Fig. 2a and 2b). The dimensions of the cavities are 8.2 × 14.7 and 9.5 × 12.0 Å for the layers based on (Mn1 + Mn2) and (Mn3 + Mn4) SBUs, respectively. The two layers are stacked alternately to generate a 3D nonporous supramolecular architecture, as shown in Fig. 2c and 2d.
 | ||
| Fig. 2 The 2D layers based on (Mn1 + Mn2) SBU (a) and (Mn3 + Mn4) SBU (b) formed by the supramolecular interactions between cages; the stacking arrangement of two layers in different colors, (c) along the c and (d) along the a axis, respectively. | ||
TGA measurement revealed that MOCC-3 can be stable up to 320 °C. The first weight loss of 15.5% from 50 to 140 °C corresponds to the loss of one uncoordinated water and six uncoordinated dmf molecules (calcd: 16%). The second weight loss of 13% from 140 to 300 °C corresponds to the loss of four coordinated dmf and four coordinated water molecules (calcd: 12.8%), and after 320 °C, MOCC-3 starts to decompose.
The temperature dependence of the molar magnetic susceptibility has been measured in the 2.0–300 K range for MOCC-3. The results are displayed in the form of χTversusT plots, T being the absolute temperature (Fig. 3). The χM value is 0.0516 cm3 mol−1 at 300 K. As temperature is lowered, the χM value increases gradually and reaches a maximum of 0.3996 cm3 mol−1 at 9 K and then decreases to 0.2868 cm3 mol−1 at 2 K, which indicates small antiferromagnetic coupling between the manganese centers.13 The χMT value is 15.65 cm3 mol−1 K at 300 K, which decreases gradually as temperature decreases, reaching 0.29 cm3 mol−1 K at 2 K. To determine the magnitude of the exchange interaction, the χvsT data were fitted by least squares using the following equation:
| χdi = [Ng2β2S(S + 1)/(kT)] (A/B) |
| A = 110exp(12.5J/kT) + 60exp(2.5J/kT) + 28exp(−5.5J/kT) + 10exp(−11.5J/kT) + 2exp(−15.5J/kT) |
| B = 11exp(12.5J/kT) + 9exp(2.5J/kT) + 7exp(−5.5J/kT) + 5exp(−11.5J/kT) + 3exp(−15.5J/kT) + exp(−17.5J/kT); |
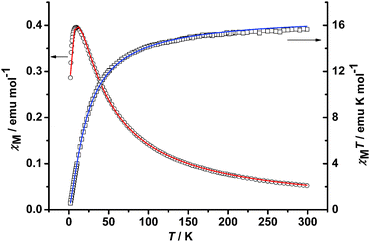 | ||
| Fig. 3 Experimental χMversusT and χMTversusT curves for MOCC-3. | ||
In conclusion, the first use of a newly-developed flexible bent dicarboxylate ligand for an assembly with the manganese ion resulted in the formation of MOCC-3 possessing a discrete cage structure with a binuclear manganese SBU as the vertex. The supramolecular interactions between the cages further connect the discrete cages into a 3D supramolecular architecture. To the best of our knowledge, MOCC-3 represents the first metal–organic coordination cage constructed from a flexible dicarboxylate ligand and a binuclear manganese SBU, although many metal–organic complexes based on a binuclear manganese SBU have been reported.13,14 Further studies will focus on the synthesis of other novel metal–organic coordination cages with this new dicarboxylate ligand.
This work was supported by the NSF of China (Grant Nos. 20701025, 20601025) and the start-up fund of Shandong University. We thank Dr Daqiang Yuan for help with magnetic discussion.
References
- (a) S. R. Batten and R. Robson, Angew. Chem., Int. Ed., 1998, 37, 1461 CrossRef; (b) S. Leininger, B. Olenyuk and P. J. Stang, Chem. Rev., 2000, 100, 853 CrossRef CAS; (c) M. H. Keefe, K. D. Benkstein and J. T. Hupp, Coord. Chem. Rev., 2000, 205, 201 CrossRef CAS; (d) M. Eddaoudi, D. B. Moler, B. Chen, T. M. Reineke, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Rev., 2001, 34, 319 Search PubMed; (e) M. Fijita and K. Ogura, Coord. Chem. Rev., 1996, 148, 249 CrossRef CAS.
- (a) Y. Cui, H. L. Ngo and W. B. Lin, Inorg. Chem., 2002, 41, 1033 CrossRef CAS; (b) P. J. Hagrman, D. Hagrman and J. Zubieta, Angew. Chem., Int. Ed., 1999, 38, 2638 CrossRef; (c) M. J. Zaworotko and B. Moulton, Chem. Rev., 2001, 101, 1629 CrossRef CAS; (d) L. Pan, B. Parker, X. Huang, D. H. Olson, J. Y. Lee and J. Li, J. Am. Chem. Soc., 2006, 128, 4180 CrossRef CAS; (e) G. Ferey, C. Mellot-Draznieks, C. Serre and F. Millange, Acc. Chem. Res., 2005, 38, 217 CrossRef CAS.
- (a) S. Kitagawa, R. Kitaura and S. -I. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef CAS; (b) J. S. Seo, D. Whang, H. Lee, S. I. Jun, J. Oh, J. Young and K. Kim, Nature, 2000, 404, 982 CrossRef CAS; (c) D. Fiedler, D. H. Leung, R. G. Bergman and K. N. Raymond, Acc. Chem. Soc., 2005, 38, 349 Search PubMed; (d) L. R. MacGillivray and J. L. Atwood, J. Solid State Chem., 2000, 152, 199 CrossRef CAS.
- (a) M. Eddaoudi, J. Kim, J. B. Wachter, H. K. Chae, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2001, 123, 4368 CrossRef CAS; (b) Z. Ni, A. Yassar, T. Antoun and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 12752 CrossRef CAS; (c) H. Furukawa, J. Kim, K. E. Plass and O. M. Yaghi, J. Am. Chem. Soc., 2006, 128, 8398 CrossRef CAS.
- (a) J. Lu, A. Mondal, B. Moulton and M. J. Zaworotko, Angew. Chem., Int. Ed., 2001, 40, 2113 CrossRef CAS; (b) B. Moulton, J. Lu, A. Mondal and M. J. Zaworotko, Chem. Commun., 2001, 863 RSC; (c) S. A. Boume, J. Lu, A. Mondal, B. Moulton and M. J. Zaworotko, Angew. Chem., Int. Ed., 2001, 40, 2111 CrossRef CAS.
- B. F. Abrahams, S. J. Egan and R. J. Robson, J. Am. Chem. Soc., 1999, 121, 3535 CrossRef CAS.
- (a) D. H. Leung, D. Fiedler, R. G. Bergman and K. N. Raymond, Angew. Chem., Int. Ed., 2004, 43, 963 CrossRef CAS; (b) M. Ziegler, J. J. Miranda, U. N. Andersen, D. W. Johnson, J. A. Leary and K. N. Raymond, Angew. Chem., Int. Ed., 2001, 40, 733 CrossRef CAS; (c) X. K. Sun, D. W. Johnson, D. L. Caulder, R. E. Powers, K. N. Raymond and E. H. Wong, Angew. Chem., Int. Ed., 1999, 38, 1303 CrossRef CAS; (d) D. L. Caulder, R. E. Powers, T. N. Parac and K. N. Raymond, Angew. Chem., Int. Ed., 1998, 37, 1840 CrossRef CAS; (e) D. Fiedler, R. G. Bergman and K. N. Raymond, Angew. Chem., Int. Ed., 2004, 43, 6748 CrossRef CAS.
- (a) M. Yoshizawa, M. Tamura and M. Fujita, Science, 2006, 312, 251 CrossRef CAS; (b) M. Fujita, N. Fujita, K. Ogura and K. Yamaguchik, Nature, 1999, 400, 52 CrossRef CAS.
- M. Schweiger, T. Yamamoto, P. J. Stang, D. Blaser and R. Boese, J. Org. Chem., 2005, 70, 4861 CrossRef CAS.
- M. C. Hong, Y. J. Zhao, W. P. Su, R. Cao, M. Fujita, Z. Y. Zhou and A. S. C. Chan, J. Am. Chem. Soc., 2000, 122, 4819 CrossRef CAS.
- (a) F. A. Cotton, P. Lei, C. Lin, C. A. Murillo, X. P. Wang, S. Y. Yu and Z. X. Zhang, J. Am. Chem. Soc., 2004, 126, 1518 CrossRef CAS; (b) R. G. Harrison, J. L. Burrows and N. K. Dalley, Inorg. Chim. Acta, 2003, 351, 399 CrossRef CAS.
- (a) C. Yang and W. T. Wong, Chem. Lett., 2004, 33, 856 CrossRef CAS; (b) C. Yang, W. T. Wong, X. M. Chen, Y. D. Cui and Y. S. Yang, Sci. Chin. B, 2003, 46, 558 Search PubMed.
- P. S. Mukherjee, S. Konar, E. Zangrando, T. Mallah, J. Ribas and N. R. Chaudhuri, Inorg. Chem., 2003, 42, 2695 CrossRef CAS.
- (a) B. Albela, M. Corbella, J. Ribas, I. Castro, J. Sletten and H. Stoeckli-Evans, Inorg. Chem., 1998, 37, 788 CrossRef CAS; (b) M. Nakashima, H. Maruo, T. Hata and T. Tokii, Chem. Lett., 2000, 1277; (c) A. Caneschi, F. Ferraro, D. Gatteschi, M. C. Melandri, P. Rey and R. Sessoli, Angew. Chem., Int. Ed. Engl., 1989, 28, 1365 CrossRef; (d) B. H. Ye, T. Mak, I. D. Williams and X. Y. Li, Chem. Commun., 1997, 1813 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: The coordination environment of central metal ions, TGA figures and crystal data for MOCC-3. CCDC reference number 702070. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b816015p |
‡ Synthesis of MOCC-3: H2L (0.02 g, 0.04 mmol) and Mn(OAc)2·4H2O (0.02 g, 0.08 mmol) were dissolved in 16 mL mixture of dmf, ethanol and H2O (v/v = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), to which two drops of pyridine were added with stirring. The solution was filtered and the filtrate was allowed to evaporate at room temperature for 2 d to give rise to a large amount of colorless block crystals of MOCC-3 (yield: 55%). Elemental analysis calcd (%) for MOCC-3: C 54.92, H 5.96, N 4.93, S 9.02; found: C 54.18, H 6.04, N 4.91, S 9.31%. 1), to which two drops of pyridine were added with stirring. The solution was filtered and the filtrate was allowed to evaporate at room temperature for 2 d to give rise to a large amount of colorless block crystals of MOCC-3 (yield: 55%). Elemental analysis calcd (%) for MOCC-3: C 54.92, H 5.96, N 4.93, S 9.02; found: C 54.18, H 6.04, N 4.91, S 9.31%. |
| § Crystal data for MOCC-3: C124H152Mn4N8O28S8, M = 2678.78, monoclinic, space groupP21/c, a = 23.922(2), b = 23.154(2), c = 28.130(3) Å, β = 100.444(2)°, U = 15323(3) Å3, Z = 4, Dc = 1.161 Mg m−3, µ(Mo Kα) = 0.485 mm−1, T = 273 K, 90283 reflections collected. Refinement of 34664 reflections (1369 parameters) with I >1.5σ(I) converged at final R1 = 0.0701, wR2 = 0.1866, gof = 0.850. CCDC 702070. The solvent molecules (four dmf molecules) in MOCC-3 are highly disordered, and attempts to locate and refine them were unsuccessful. The SQUEEZE program was used to remove scattering from the highly disordered solvent molecules and a new.HKL file was generated. The structure was solved by using the new generated.HKL file. Quality values for the unmodified data: R1 = 0.1286, wR2 = 0.3543, gof = 1.255. |
| This journal is © The Royal Society of Chemistry 2009 |
