[Yb(C2O4)4]5− – a versatile metal–organic building block for layered coordination polymers†‡
Ulrich
Baisch
and
Dario
Braga
*
Dipartimento di Chimica G. Ciamician, Università di Bologna, Via F. Selmi 2, 40126, Bologna, Italy. E-mail: dario.braga@unibo.it; Fax: +39-051-209-9456; Tel: +39-051-209-9555
First published on 24th October 2008
Abstract
A crystal engineering approach affords the novel crystalline coordination polymer [C3H7N6]6[Yb(C2O4)4][NO3] (1) based on the interaction between the monomeric building-block [Yb(C2O4)4]5− and melamine creating a new structural motif in lanthanide chemistry with prospects for electromagnetic properties.
Rare earth compounds belong to one of the most applicative branches in chemistry because of the wide variety of properties shown by their solids. Materials with outstanding properties have already been reported, e.g. the ability to bind and release gases, superconductivity, and luminescence.1–6 On the other hand, crystal engineering of rare earth containing metal–organic networks is often met with intrinsic difficulties because of the high coordination numbers and a clear preference to form large ions.1,2 Besides, the very oxophilic character of the rare earth elements often leads to the coordination of water to the lanthanide centre, thus inhibiting the assembly of coordination networks.1,2 On the other hand, synthesis in non-aqueous systems brings about difficulties for a further industrial approach and decreases significantly the applicative character of this field of research. A way out has often been shown to be the use of higher pressure conditions. Indeed, a vast number of supramolecular complexes were published in the last years synthesised under hydrothermal conditions.7–9 Strong coordinating sterically demanding ligands sometimes offer alternatives. They saturate the ligand sphere and, depending on the nature of the ligand, may build up MOF's or networks.1,3,10–12 Following this concept, oxalic acid is both a versatile ligand and a linker molecule13,14 with a strongly binding character to the lanthanide centre due to its chelating nature.
Our scope is the engineering of crystal structures where organic and inorganic carboxylic acids form networks built up by strong or medium hydrogen bonding with organic tectons.15–23 In an attempt to expand theses strategies to lanthanides, we report here the result of the combination of the coordinating character of anionic water-free oxalato lanthanides with the tripodal symmetry of protonated melamine which allowed us to engineer crystals consistent with a layered coordination polymer. The chelating oxalate ligand plays the role of a linker molecule between melaminium layers simultaneously saturating the ligand sphere of the lanthanide ion. We describe the results of the first synthesis and crystal structure determination of such a layer-like metal–organic coordination polymer consistent of mononuclear water-free tetraoxalato ytterbium(III) units, monoprotonated melamine, and nitrate.
The compound has been synthesized by suspending commercially available reagents in boiling water. Addition of a hot solution of mel (mel = melamine) to a hot suspension of freshly prepared ytterbium oxalate (Yb(NO3)3·5H2O and oxalic acid) followed by refluxing the mixture for three more days resulted in the formation of a crystalline colourless solid. Single-crystal X-ray analysis of suitable crystals found in the solid revealed a structure with composition [Hmel]6[Yb(C2O4)4][NO3]·(H2O)3.65, 1. The purity of the recovered solid was controlled by XPD analysis (XPD = X-ray power diffraction). Both patterns—the experimental and the one derived from single-crystal data—coincide perfectly (Fig. 1).
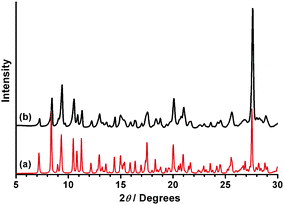 | ||
| Fig. 1 Comparison of calculated (a) and measured (b) X-ray powder diffraction patterns of 1. | ||
The structure of 1 possesses some remarkable features. In the first instance the tetraoxalato ytterbate(III) units have a formal charge of −5, which is rarely encountered for lanthanide coordination networks in the literature.24,25 In fact, one nitrate anion is included into the structure increasing the formal overall charge in the unit cell to −6 with six Hmel molecules as counter-ions. To the best of our knowledge our tetra-oxalato anion [Yb(C2O4)4]5− is the first example of a crystallographically characterised isolated water-free lanthanide tetraoxalate complex. Usually, solvent-free lanthanide ions are linked to each other by bridged bis-chelating oxalate ions.14,26–28 Furthermore, the ytterbium ion is at the centre of a very regular cubic antiprismatic coordination polyhedron, which is more typical for solvated lanthanide ions29–31 and not of a bicapped trigonal prismatic, which is much more common for these chelating ligands.14,26–28 Both anions, nitrate and [Yb(C2O4)4]5− are arranged in an alternate manner, one over the other, connecting the planes of Hmel to each other (Fig. 2).
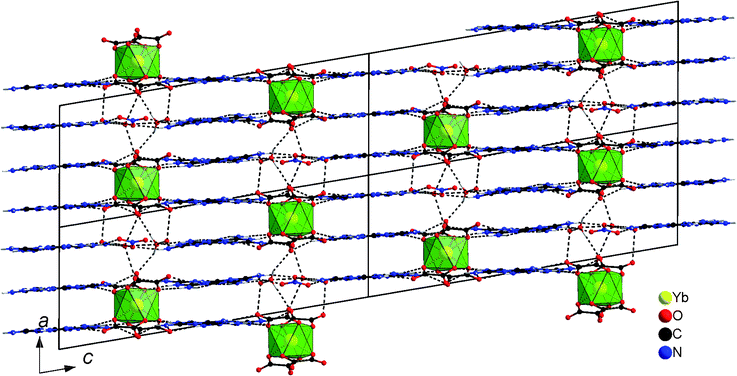 | ||
| Fig. 2 Crystal structure of 1 with a view of the ac plane, dashed lines depict hydrogen bonding. | ||
Where nitrate is present in the structure solvent, water molecules fill the residual cavities showing O(H)⋯O and N(H)⋯O contacts in the range 2.56–3.15 Å and 2.62–2.97 Å, respectively. Both anionic tectons are connected to mel by a large number of hydrogen bridges with distances N(H)⋯O between 2.74 and 3.10 Å. Additionally, Hmel molecules are connected by strong N–H⋯N hydrogen bonds (N⋯O = 2.92–3.07 Å) forming zig-zag planes on the ac plane. Fig. 3 depicts one Hmel plane including both anionic building blocks and hydrogen bonds in top view.
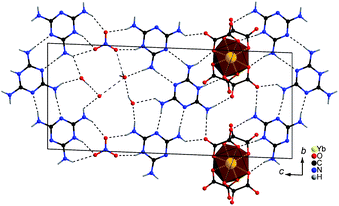 | ||
| Fig. 3 One plane of the layered crystal structure of 1 depicted in top view on the bc plane, dashed lines depict hydrogen bonding. | ||
Melaminium seems to play a structure-determining role in this structure. The zig-zag arrangement of protonated melamine is well known in the literature32–41 with up to three parallel hydrogen bonds connecting each Hmel to the next. Indeed, compared with already reported lanthanide tetraoxalate structures the penta-anion observed in 1 seems to be much more flattened and aligned.14,26–28 This effect can be explained as a consequence of the strong molecular interactions to Hmel, nitrate, and water forcing [Yb(C2O4)4]5− into shape.42 However, an even more surprising result was the migration of NO3− out of the ligand sphere of the ytterbium ion into the alternate position below and above the anion [Yb(C2O4)4]5−. Before conducting the experiment we expected a formation of an ytterbium oxalate network, as reported by Mohanu et al.14 with a formal charge of −2 or −4. With using a planar trigonal base such as mel, the crystallization conditions change much due to the low solubility of this aromatic tecton and its strong hydrogen bonding character. In solution ytterbium oxalate will be monomeric.24,25 Thus, a fast crystallization combined with the particular chemical properties of melamine will be responsible for the composition of 1. Seen from this point of view a participation of nitrate anions still present in the solution is a logical consequence of reaching charge equalisation. Solvent water was introduced as molecular padding to fill up the space resulting from the sterical discrepancy between [Yb(C2O4)4]5− and NO3−. Further synthesis and crystallizations are in progress using different lanthanides such as europium, gadolinium, cerium. The solids obtained did already show similar XPD patterns. Additionally, promising preliminary results regarding special optical and magnetic properties have been obtained. The corresponding europium and gadolinium compound did show luminescence under UV-light (λ = 254 nm). More detailed analysis will be reported in due course.
In summary, we have provided evidence for a convenient “green” synthesis to a new rare earth containing crystalline layered 3D coordination polymer involving inexpensive reagents of great use and importance in industry and fundamental research: oxalic acid and melamine.
We gratefully acknowledge the help of Elena Mercolini with some of the experiments and thank MIUR (PRIN 2007) and the University of Bologna for financial support. Furthermore, this research was supported by a Marie Curie Intra-European Fellowship within the 7th European Community Framework Program.
References
- J.-C. G. Buenzli, Acc. Chem. Res., 2006, 39, 53–61 CrossRef.
- J.-C. G. Buenzli and C. Piguet, Chem. Rev., 2002, 102, 1897–1928 CrossRef.
- R. J. Hill, D.-L. Long, P. Hubberstey, M. Schroeder and N. R. Champness, J. Solid State Chem., 2005, 178, 2414–2419 CrossRef.
- T. K. Maji, G. Mostafa, H.-C. Chang and S. Kitagawa, Chem. Commun., 2005, 2436–2438 RSC.
- S. K. Ghosh, J.-P. Zhang and S. Kitagawa, Angew. Chem., Int. Ed., 2007, 46, 7965–7968 CrossRef CAS.
- D. Tanaka and S. Kitagawa, Chem. Mater., 2008, 20, 922–931 CrossRef CAS.
- J. Lu, W.-H. Bi, F.-X. Xiao, S. R. Batten and R. Cao, Chem.–Asian J., 2008, 3, 542–547 CrossRef CAS.
- F. Luo, S. R. Batten, Y. Che and J.-M. Zheng, Chem.–Eur. J., 2007, 13, 4948–4955 CrossRef CAS.
- J. Xia, B. Zhao, H.-S. Wang, W. Shi, Y. Ma, H.-B. Song, P. Cheng, D.-Z. Liao and S.-P. Yan, Inorg. Chem., 2007, 46, 3450–3458 CrossRef CAS.
- J. Jia, X. Lin, J. Blake Alexander, R. Champness Neil, P. Hubberstey, L. Shao, G. Walker, C. Wilson and M. Schroder, Inorg. Chem., 2006, 45, 8838–8840 CrossRef CAS.
- D.-L. Long, A. J. Blake, N. R. Champness, C. Wilson and M. Schroeder, J. Am. Chem. Soc., 2001, 123, 3401–3402 CrossRef CAS.
- D.-L. Long, R. J. Hill, A. J. Blake, N. R. Champness, P. Hubberstey, D. M. Proserpio, C. Wilson and M. Schroeder, Angew. Chem., Int. Ed., 2004, 43, 1851–1854 CrossRef CAS.
- H. Kara, C. J. Adams, A. G. Orpen and T. J. Podesta, New J. Chem., 2006, 30, 1461–1469 RSC.
- A. Mohanu, C. Brouca-Cabarrecq and J. C. Trombe, J. Solid State Chem., 2006, 179, 3–17 CrossRef CAS.
- D. Braga, M. Cadoni, F. Grepioni, L. Maini and J. van de Streek, New J. Chem., 2007, 31, 1935–1940 RSC.
- D. Braga, M. Curzi, F. Grepioni and M. Polito, Chem. Commun., 2005, 2915–2917 RSC.
- D. Braga, S. L. Giaffreda, F. Grepioni, M. R. Chierotti, R. Gobetto, G. Palladino and M. Polito, CrystEngComm, 2007, 9, 879–881 RSC.
- D. Braga, S. L. Giaffreda, F. Grepioni, L. Maini and M. Polito, Coord. Chem. Rev., 2006, 250, 1267–1285 CrossRef CAS.
- D. Braga, S. L. Giaffreda, M. Polito and F. Grepioni, Eur. J. Inorg. Chem., 2005, 2737–2746 CrossRef.
- D. Braga and L. Maini, Chem. Commun., 2004, 976–977 RSC.
- D. Braga, M. Polito and F. Grepioni, Cryst. Growth Des., 2004, 4, 769–774 CrossRef CAS.
- D. Braga, K. Rubini and L. Maini, CrystEngComm, 2004, 6, 236–238 RSC.
- R. Gobetto, C. Nervi, M. R. Chierotti, D. Braga, L. Maini, F. Grepioni, R. K. Harris and P. Hodgkinson, Chem.–Eur. J., 2005, 11, 7461–7471 CrossRef CAS.
- J. Dervin, J. Dumonceau, F. Fromage and J. Faucherre, Mater. Chem. Phys., 1983, 8, 109–118 CrossRef CAS.
- J. Dumonceau, F. Fromage and J. Faucherre, Bull. Soc. Chim. Fr., 1981, 319–323 CAS.
- I. A. Kahwa, F. R. Fronczek and J. Selbin, Inorg. Chim. Acta, 1984, 82, 161–166 CrossRef CAS.
- I. A. Kahwa, F. R. Fronczek and J. Selbin, Inorg. Chim. Acta, 1984, 82, 167–172 CrossRef CAS.
- T. R. R. McDonald and J. M. Spink, Acta Crystallogr., 1967, 23, 944–949 CrossRef CAS.
- A. E. Clark, J. Chem. Theory Comput., 2008, 4, 708–718 CrossRef CAS.
- I. Persson, P. D'Angelo, S. De Panfilis, M. Sandstrom and L. Eriksson, Chem.–Eur. J., 2008, 14, 3056–3066 CrossRef CAS.
- X. Zhu, Z. Ma, W. Bi, Y. Wang, D. Yuan and R. Cao, CrystEngComm, 2008, 10, 19–22 RSC.
- J. Janczak and J. Perpetuo Genivaldo, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2002, 58, o455–459 CrossRef.
- J. Janczak and J. Perpetuo Genivaldo, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2003, 59, o349–352 CrossRef.
- J. Janczak and G. J. Perpetuo, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2001, C57, 1431–1433 CrossRef CAS.
- J. Janczak and G. J. Perpetuo, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2001, 57, 1120–1122 CrossRef CAS.
- H. Jing, M. Stroebele, M. Weisser and H. J. Meyer, Z. Anorg. Allg. Chem., 2003, 629, 368–370 CrossRef CAS.
- M. K. Marchewka, J. Janczak, S. Debrus, J. Baran and H. Ratajczak, Solid State Sci., 2003, 5, 643–652 CrossRef CAS.
- B. V. Lotsch and W. Schnick, Chem. Mater., 2006, 18, 1891–1900 CrossRef CAS.
- B. V. Lotsch and W. Schnick, Z. Anorg. Allg. Chem., 2007, 633, 1435–1441 CrossRef CAS.
- Y. Sugiyama, K. Adachi, S. Kawata, H. Kumagai, K. Inoue, M. Katada and S. Kitagawa, CrystEngComm, 2000 Search PubMed No pp given, Article No 32.
- M. K. Kabir, H. Tobita, H. Matsuo, K. Nagayoshi, K. Yamada, K. Adachi, Y. Sugiyama, S. Kitagawa and S. Kawata, Cryst. Growth Des., 2003, 3, 791–798 CrossRef CAS.
- A. G. Orpen, L. Brammer, F. H. Allen, O. Kennard, D. G. Watson and R. Taylor, J. Chem. Soc., Dalton Trans., 1989, S1–S83 RSC.
- G. M. Sheldrick, SHELXS97, Program for Crystal Structure solution, 1997, University of Göttingen, Göttingen, Germany Search PubMed.
- G. M. Sheldrick, SHELXL97, Program for Crystal Structure refinement, 1997, University of Göttingen, Göttingen, Germany Search PubMed.
- K. Brandenburg, DIAMOND, Programm for X-ray structure analysis, 2008, Crystal Impact GbR, Bonn, Germany Search PubMed.
Footnotes |
| † CCDC reference number 693612. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b816022h |
| ‡ All starting materials or solvents were purchased from Sigma-Aldrich (acetone, oxalic acid dihydrate, nitric acid) or Alfa Aesar (Yb2O3, melamine) and used without any further purification. Reagent grade solvents and bidistilled water were used as received. Yb(NO3)3·5H2O was synthesized dissolving Yb2O3 in diluted nitric acid in a round bottom flask at 90 °C followed by evaporation to dryness at 60 °C. |
| This journal is © The Royal Society of Chemistry 2009 |
