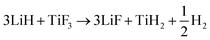Improved hydrogen storage property of Li–Mg–B–H system by milling with titanium trifluoride†
Peijun Wang, Laipeng Ma, Zhanzhao Fang, Xiangdong Kang and Ping Wang*
Shenyang National Laboratory for Materials Science, Institute of Metal Research, Chinese Academy of Sciences, 72 Wenhua Road, Shenyang, 110016, P.R., China. E-mail: pingwang@imr.ac.cn; Fax: +86 24 2389 1320; Tel: +86 24 2397 1622
First published on 7th November 2008
Abstract
The Li–Mg–B–H system that is prepared from 2LiH + MgB2 or 2LiBH4 + MgH2 possesses high hydrogen capacity and relatively favorable thermodynamics, but it is greatly restricted in practical hydrogen storage applications by problematic H-exchange kinetics. In the present study, TiF3 was mechanically milled with a 2LiH + MgB2 mixture and examined with respect to its effect on reversible dehydrogenation of the Li–Mg–B–H system. Experimental study showed that TiF3 is highly effective for promoting the two-step dehydrogenation reaction in the Li–Mg–B–H system. Compared to the neat 2LiH + MgB2 sample, the 2LiH + MgB2 + 0.01TiF3 sample exhibits significantly reduced dehydrogenation temperature and markedly enhanced dehydriding rate at both steps. Furthermore, the catalytic enhancement arising upon adding TiF3 additive was observed to persist well in the hydrogenation/dehydrogenation cycles. Based on the results of phase analysis and a series of designed experiments, the mechanism underlying the observed property improvement is discussed.
Broader contextOn-board hydrogen storage has been generally recognized as a key technical challenge in promoting the commercialization of hydrogen-powered vehicles. The Li–Mg–B–H system has attracted considerable interest as a potential hydrogen storage medium due to its high H-capacity (with a theoretical value of 11.8 wt%) and relatively good thermodynamics. Its application is, however, greatly hindered by problematic H-exchange kinetics. In the present paper, we report our latest findings regarding the effect of titanium trifluoride (TiF3) on the reversible dehydrogenation properties of the Li–Mg–B–H system. It was found that mechanically milling with TiF3 additive resulted in, significantly lowered dehydrogenation temperature and markedly improved dehydriding kinetics of the Li–Mg–B–H system. Moreover, the property enhancement arising upon adding TiF3 persists well in the re-/dehydrogenation cycles. |
1. Introduction
On-board hydrogen storage has been generally recognized as a key technical challenge in promoting the commercialization of hydrogen-powered vehicles. Decades of extensive research efforts on various hydrogen storage materials have led to no viable system that can reversibly store 5 kg hydrogen (to enable a driving range >300 miles without refueling) within the vehicular constraints of weight, volume, efficiency, safety, durability and cost.1–6 Recently, lithium borohydride (LiBH4) has received considerable attention as a potential hydrogen storage media due to its extremely high hydrogen content. Several novel approaches have been developed to address its problematic H-exchange kinetics and thermodynamics, including reactant destabilization, cation/anion substitution, and tailored nanophase structure using nanoporous scaffolds.7–13 As a result of these technical advances, several material systems that possess superior hydrogen storage property to the parent LiBH4 have been identified. One prominent example is the Li–Mg–B–H system.Vajo et al. demonstrated that LiBH4 can be effectively destabilized by mechanical milling with 1/2MgH2.14–16 The newly constituted Li–Mg–B–H system undergoes reversible dehydrogenation/rehydrogenation reactions following eqn (1).
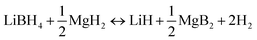 | (1) |
As a result of the stabilized dehydrogenated state, the reaction enthalpy of the Li–Mg–B–H system is reduced by about 25 kJ mol−1 H2, compared with pure LiBH4.17 Furthermore, the significant thermodynamic improvement is achieved at only a moderate capacity cost. The theoretical hydrogen capacity of the Li–Mg–B–H system is still as high as 11.8 wt%, which justifies further efforts to assess its potential for hydrogen storage applications. Due to the kinetic limitation as well as the lack of effective catalyst, the Li–Mg–B–H system requires a temperature of over 400 °C to fulfill dehydrogenation at a reasonable rate. This is much higher than that predicted using thermodynamic data (around 225 °C).14
In our recent studies on the light-metal complex hydrides (NaAlH4, LiBH4) and metal hydride (MgH2) systems, we found that titanium trifluoride (TiF3) was superior to its analogue titanium trichloride (TiCl3) as a catalytic additive.18–24 Experimental studies found that the materials with TiF3 additive always exhibited more favorable kinetic performance than those samples with TiCl3 additive. Theoretical calculations showed that fluorine (F)-doping in the complex hydrides may cause lattice substitution of H by F anion, and accordingly result in favorable thermodynamic modification.25 Motivated by these experimental and theoretical findings, we further extended the usage of TiF3 additive in the Li–Mg–B–H system. Again, our study results demonstrated its effectiveness. The addition of catalytic amounts of TiF3 was found to result in significantly improved dehydrogenation kinetics in the Li–Mg–B–H system. Furthermore, the catalytic enhancement arising upon adding TiF3 persists well in the de-/rehydrogenation cycles. We herein report the study results.
2. Experimental
The starting materials LiH (95%), TiF3, TiCl3 (99.999%), TiH2 (98%) were all purchased from Sigma-Aldrich Corp. MgB2 (325 mesh) was purchased from Alfa Aesar Corp. All the materials were used as received.The 2LiH + MgB2 mixture was mechanically milled under Ar atmosphere for 5 h using a Fritsch 7 Planetary mill at 400 rpm in a stainless steel vial together with eight steel balls (10 mm in diameter). For comparison, the samples with catalytic additive (TiF3, TiCl3, LiF or TiH2) were mechanically prepared under identical conditions to that applied for the neat 2LiH–MgB2 sample. The prepared samples were denoted as 2LiH–MgB2–xTiF3 (TiCl3, LiF or TiH2), where x is the mole ratio of the catalytic additive relative to MgB2. The ball-to-powder ratio was around 40 : 1. All sample operations were performed in an Ar (99.999%)-filled glovebox, which was equipped with a recirculation system to keep the H2O and O2 levels below 0.1 ppm.
Hydriding/dehydriding properties of the samples were examined using a carefully calibrated Sievert's type apparatus. A typical cyclic experiment entailed dehydrogenation at 400 °C and hydrogenation at 350 °C with an initial hydrogen pressure of 0.3 and 7.5 MPa, respectively. To minimize H2O/O2 contamination during the volumetric measurements, the hydrogen feed (with an initial purity of 99.999%) was further purified using a hydrogen storage alloy system. For comparison with the neat sample, the weights of TiF3 or other catalytic additives were not taken into account in determination of H-capacity.
The samples in the hydrogenated state were also examined by synchronous thermal analysis (thermogravimetry/differential scanning calorimetry/mass spectrometry, TG/DSC/MS, Netzsch 449C Juptier/QMS 403C). In the thermal analysis measurements, high-purity Ar (99.999%) was used as the purge gas. The heating rate was 5 °C min−1. The samples for thermal analysis measurement (with a typical amount of around 4 mg) were put into a crucible in the glovebox. The subsequent sample transferring into TG/DSC/MS equipment was performed in a specially designed Ar-filled device.
The samples were characterized by powder X-ray diffraction (XRD, Rigaku D/MAX-2500, Cu Kα radiation), X-ray photoelectron spectroscopy (XPS, VG ESCALAB 250, Al Kα radiation) and scanning electron microscopy (SEM, LEO Supra 35) equipped with an energy dispersive X-ray (EDX) analyzer unit (Oxford). All the sample preparations were performed in the glovebox. To minimize the H2O/O2 contamination during the XRD measurement, a small amount of grease was used to cover the sample surface. In the XPS measurements, binding energies of all the samples were measured using the C 1s peak (284.6 eV) of the adventitious carbon as an internal standard. Special measures were taken to minimize H2O/O2 contamination during sample transfer from the glove-box into the XPS chamber.
3. Results and discussion
Reversible dehydrogenation of the Li–Mg–B–H system occurs via a two-step process involving the reactions shown in eqn (2) and (3). The first step releases 0.5 equiv. of H2via decomposition of MgH2, and the second step yields another 1.5 equiv. of H2 through the reaction between LiBH4 and Mg.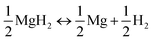 | (2) |
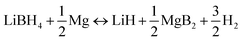 | (3) |
Our study found that both dehydrogenation steps can be significantly promoted by adding small amount of Ti halides. Fig. 1 presents the DSC profiles of the hydrogenated 2LiH–MgB2 samples with and without halide additive (TiF3 or TiCl3), respectively. In all the three samples, three endothermic peaks can be clearly identified. The first one originates from the melting of LiBH4. The other two at higher temperature correspond to the dehydrogenation reactions (2) and (3), respectively. Compared with the neat sample, the samples with TiF3 or TiCl3 additive show significantly reduced dehydrogenation temperature in both steps. Particularly, the 2LiH–MgB2–0.01TiF3 sample exhibits peak temperatures at 309 °C and 388 °C, which are 56 °C and 106 °C lower than the corresponding values of neat 2LiH–MgH2 sample, respectively. The observed advantage of TiF3 over its analogue TiCl3 as a catalytic additive is consistent with our previous findings in sodium alanate (NaAlH4) and magnesium hydride (MgH2) systems, suggesting that the halide anion may also contribute to the property improvement. Additionally, the 2LiH–MgB2–0.01TiF3 sample exhibits a melting peak at 262 °C, which is significantly lower than those observed in the neat 2LiH–MgH2 and 2LiH–MgB2–0.01TiCl3 samples. Currently, the mechanism underlying this interesting phenomenon is still unclear. Presumably, it might be associated with F-substitution in the LiBH4 lattice, which has been theoretically elucidated to be an energetically favorable process.25
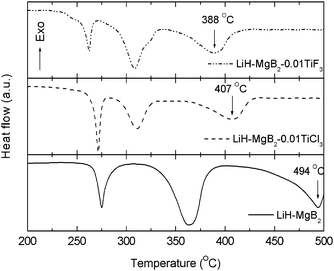 | ||
| Fig. 1 Comparison of the DSC profiles of neat 2LiH–MgB2, 2LiH–MgB2–0.01TiF3 and 2LiH–MgB2–0.01TiCl3 samples. All the three samples were previously hydrogenated at 350 °C under an initial hydrogen pressure of 7.5 MPa for 12 h. The ramping rate is 5 °C min−1. | ||
The pronounced effect of TiF3 in promoting the dehydrogenation reactions of the Li–Mg–B–H system is further demonstrated in isothermal volumetric measurements. Fig. 2 presents a comparison of the dehydriding kinetics between samples with and without TiF3 additive, respectively. For both samples, the dehydriding curves show a typical two-step dehydrogenation feature. It is noteworthy that the initial flat sections in all the curves do not reflect the intrinsic dehydriding property of the samples, but an artifact resulting from the measuring method. In the isothermal measurements, data collection started from the moment of pushing the reactor into the furnace, which was held at desired temperatures. A temperature-rising period is therefore required for initiating the dehydrogenation reaction of the samples. Under identical measurement conditions, the duration of the flat section becomes shorter upon adding TiF3, indicative of its effect on promoting the dehydrogenation of MgH2. Notably, an even more pronounced property improvement was observed at the second dehydrogenation step. In the second dehydrogenation step, the hydrogenated 2LiH–MgB2–0.01TiF3 sample rapidly releases 5.5 wt% hydrogen within 20 min at 400 °C, with an average dehydrogenation rate nearly one order of magnitude higher than that of neat 2LiH–MgB2 sample. To the best of our knowledge, such a pronounced property improvement has never been achieved in the Li–Mg–B–H system using other catalyst additives.14,17 For example, compared with previously reported catalysts such as titanium isopropoxide, TiF3 additive leads to four times faster reaction kinetics with only one fifth of the mole ratio that is added, yet it releases the same weight amount of hydrogen.17 Further study indicates that the catalytic enhancement arising upon adding TiF3 persists well in the hydrogenation/dehydrogenation cycles. As shown in Fig. 2, the recycled 2LiH–MgB2–0.01TiF3 sample exhibits well maintained kinetics and only a slight capacity loss, as compared to the performance in the first cycle. As the hydrogenation process was performed under high hydrogen pressure (with an initial value of 7.5 MPa), a small temperature variation resulted in considerable pressure fluctuation. But, judging from the pressure change, the samples with TiF3 additive could largely complete hydrogenation within 4 h at 350 °C, with an average rehydriding rate three times faster than that of neat 2LiH–MgB2 samples.
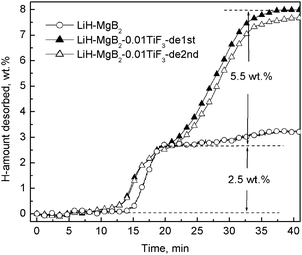 | ||
| Fig. 2 Comparison of the dehydriding (DE) curves of neat 2LiH–MgB2 and 2LiH–MgB2–0.01TiF3 samples at 400 °C. Both samples were previously hydrogenated at 350 °C under an initial hydrogen pressure of 7.5 MPa for 12 h. | ||
In our preliminary efforts to understand the promoting effect of TiF3, we firstly performed XRD phase analysis. Fig. 3 presents the XRD patterns of the 2LiH–MgB2–0.01TiF3 sample at varied states. These results confirm the reversible dehydrogenation/rehydrogenation reactions of the Li–Mg–B–H system. On the other hand, identification of the MgB2 phase in pattern (b) indicates incomplete rehydrogenation of the sample. This may qualitatively account for the capacity gap between the theoretical value (11.2 wt%) and that practically obtained (∼8 wt%). Careful examination of the XRD pattern of the post-milled sample found that the diffraction peak of LiH at 2θ = 44.4° exhibited considerable asymmetry, with the shoulder peak at the higher angle side corresponding well to LiF (shown in the lower inset). Additionally, XPS examination confirmed the formation of TiH2 in the post-milled sample (see ESI†). These results clearly indicate the occurrence of reaction (4) in the milling process.
| ΔrGm = −284 kJ mol−1.26 | (4) |
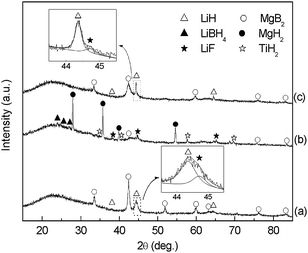 | ||
| Fig. 3 XRD patterns of the 2LiH–MgB2–0.01TiF3 sample: (a) post-milled; (b) after hydrogenation at 350 °C with an initial hydrogen pressure of 7.5 MPa; (c) after the first dehydrogenation at 400 °C. | ||
EDX analysis of the post-milled sample showed fine dispersion of Ti-containing nanoparticles in the LiH–MgB2 matrix, which is consistent with the invisibility of Ti hydride in the XRD analysis. In the rehydrogenated sample, both Ti hydride and LiF phases were clearly identified. But after the subsequent dehydrogenation reaction, the Ti hydride phase becomes hardly detectable by XRD analysis, whereas its presence was confirmed by parallel XPS examination. (see ESI†). These findings indicate that the dehydrogenation reaction of the system results in a considerable change in dispersion state or crystal structure of the Ti hydride. In this regard, further detailed study is still required. Additionally, close examination of the XRD patterns found that the relative intensity of the LiF to LiH peaks decreased after one re-/dehydrogenation cycle. This finding is consistent with our hypothesis regarding the functionality of F anion, and is discussed below.
Several designed experiments were subsequently performed to examine the effect of the newly formed phases on reversible dehydrogenation of the Li–Mg–B–H system. As can be seen from the kinetics comparison presented in Fig. 4, the addition of LiF was found to exert no appreciable influence on the dehydriding property of the Li–Mg–B–H system, while directly milling 2LiH–MgB2 with 1 mol% TiH2 powder resulted in considerable improvement of the dehydriding kinetics, but with a degree of property improvement that was inferior to that obtained in the 2LiH–MgB2–0.01TiF3 sample. These findings clearly indicate that, besides the catalytically active TiH2 phase, there exists another mechanism that may be responsible for the observed property improvement arising upon adding TiF3. One prominent possibility is the functionality of the F anion. According to our first-principle calculations, F anion is substantially different from its analogue Cl anion, which generates dead weight by-products. Doping LiBH4 with F anion may generate lattice substitution of H by F anion in both the hydrogenated (LiBH4) and dehydrogenated (LiH) states of the hydride, and accordingly result in favorable thermodynamics modification.25 Experimentally, this hypothesis has been supported by the demonstrated advantage of TiF3 over TiCl3 in improving the hydrogen storage properties of the hydride. Additionally, the abnormal melting point decrease of borohydride that was observed in the TiF3-doped sample and the decreased diffraction intensity of LiF in the recycled sample may also serves as indirect evidence. Currently, we are carrying out coupled theoretical/experimental efforts to further understand the mechanisms of the TiF3-doped Li–Mg–B–H material system.
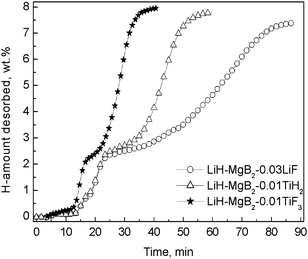 | ||
| Fig. 4 Comparison of the dehydriding (DE) curves of the 2LiH–MgB2–0.01TiF3, 2LiH–MgB2–0.01TiH2 and 2LiH–MgB2–0.03LiF composites that were previously hydrogenated at 350 °C 7.5 MPa for 12 h. | ||
Conclusions
The two-step reversible dehydrogenation properties of the Li–Mg–B–H system can be significantly improved by mechanically milling with small amount of TiF3 additive. Compared with the neat 2LiH–MgB2 sample, the sample containing 1 mol% TiF3 additive exhibited a lower dehydrogenation temperature by 56 °C and 106 °C, respectively, for the first (MgH2 → Mg) and second (LiBH4 + Mg → LiH + MgB2) dehydrogenation steps, and a nearly one order of magnitude increase on the average dehydriding rate at the second step. Furthermore, the property enhancement arising upon adding TiF3 persists well in the subsequent re-/dehydrogenation cycles. According to the results of phase analysis and designed experiments, the observed property improvement may be ascribed to the combined effects of catalytically active Ti hydride nanoparticles and the functional F anion.Acknowledgements
Financial support for this research from the Hundred Talents Project of the Chinese Academy of Sciences, the National Natural Science Foundation of China (Grants No. 50571099 and 50771094) and the special funding for CAS President Prize winner are gratefully acknowledged.References
- R. L. Cohen and J. H. Wernick, Science, 1981, 214, 1081–1087 CrossRef CAS.
- L. Schlapbach and A. Züttel, Nature, 2001, 414, 353–358 CrossRef.
- B. Bogdanović and M. Schwickardi, J. Alloys Compd., 1997, 253–254, 1–9 CrossRef CAS.
- L. Maj and W. Grochala, Adv. Funct. Mater., 2006, 16, 2061–2076 CrossRef CAS.
- P. Wang and X. D. Kang, Dalton Trans., 2008, 5400–5413 RSC.
- S. Orimo, Y. Nakamori, J. R. Eliseo, A. Züttel and C. M. Jensen, Chem. Rev., 2007, 107, 4111–4132 CrossRef CAS.
- W. Grochala and P. P. Edwards, Chem. Rev., 2004, 104, 1283–1315 CrossRef CAS.
- F. Schüth, B. Bogdanović and M. Felderhoff, Chem. Commun., 2004, 2249–2258 RSC.
- A. Züttel, S. Rentsch, P. Fischer, P. Wenger, P. Sudan, Ph. Mauron and Ch. Emmenegger, J. Alloys Compd., 2003, 356, 515–520 CrossRef.
- A. Züttel, P. Wenger, P. Rentsch, P. Sudan, Ph. Mauron and Ph. Emmenegger, J. Power Sources, 2003, 118, 1–7 CrossRef CAS.
- S. Orimo, Y. Nakamori, G. Kitahara, K. Miwa, N. Ohba, S. Towata and A. Züttel, J. Alloys Compd., 2005, 404, 427–430 CrossRef.
- Y. Nakamori, S. Orimo and T. Tsutaoka, Appl. Phys. Lett., 2006, 88, 112104 CrossRef.
- A. F. Gross, J. J. Vajo, S. L. Van Atta and G. L. Olson, J. Phys. Chem. C, 2008, 112, 5651–5657 CrossRef CAS.
- J. J. Vajo, S. L. Skeith and F. Mertens, J. Phys. Chem. B, 2005, 109, 3719–3722 CrossRef CAS.
- J. J. Vajo and G. L. Olson, Scr. Mater., 2007, 56, 829–834 CrossRef CAS.
- F. E. Pinkerton, M. S. Meyer, G. P. Meisner, M. P. Balogh and J. J. Vajo, J. Phys. Chem. C, 2007, 111, 12881–12885 CrossRef CAS.
- U. Bösenberg, S. Doppiu, L. Mosegaard, G. Barkhordarian, N. Eigen, A. Borgschulte, T. R. Jensen, Y. Cerenius, O. Gutfleisch, T. Klassen, M. Dornheim and R. Bormann, Acta Mater., 2007, 55, 3951–3958 CrossRef.
- X. D. Kang, P. Wang and H. M. Cheng, Scr. Mater., 2007, 56, 361–364 CrossRef CAS.
- P. Wang, X. D. Kang and H. M. Cheng, J. Phys. Chem. B, 2005, 109, 20131–20136 CrossRef CAS.
- P. Wang, X. D. Kang and H. M. Cheng, ChemPhysChem, 2005, 6, 2488–2491 CrossRef CAS.
- P. Wang, X. D. Kang and H. M. Cheng, J. Alloys Compd., 2006, 421, 217–222 CrossRef CAS.
- L. P. Ma, P. Wang and H. M. Cheng, J. Alloys Compd., 2007, 432, L1–4 CrossRef CAS.
- L. P. Ma, P. Wang, X. D. Kang and H. M. Cheng, J. Mater. Res., 2007, 22, 1779–1786 CrossRef CAS.
- Y. Nakamura, A. Fossdal, H. W. Brinks and B. C. Hauback, J. Alloys Compd., 2006, 416, 274–278 CrossRef CAS.
- L. C. Yin, P. Wang, Z. Z. Fang and H. M. Cheng, Chem. Phys. Lett., 2008, 450, 318–321 CrossRef CAS.
- I. Barin, Thermochemical Data of Pure Substances, Wiley-VCH Verlag GmbH, Weinheim, Germany, 3rd edn, 1995, pp. 961–966, 1681–1684 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: XPS binding energy for Ti in TiH2 species. See DOI: 10.1039/b815934c |
| This journal is © The Royal Society of Chemistry 2009 |