Production of biodiesel from side-stream refining products
Camelia
Echim
*a,
Roland
Verhé
a,
Wim
De Greyt
b and
Christian
Stevens
a
aDepartment of Organic Chemistry, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, 9000, Ghent, Belgium. E-mail: camelia.echim@UGent.be; Fax: +32 9 264 62 43; Tel: +32 9 264 59 54
bDesmet Ballestra Group, Minervastraat 1, 1930, Zaventem, Belgium
First published on 18th September 2009
Abstract
The main focus of this review is to offer an overview of different existing processes for converting side-stream refining products (soapstock, acid oil and deodorized distillates) into new generation energy sources. The review article calls for more research around the SSRPs for its efficient conversion into biodiesel/biofuel and outlines drawbacks of the methodologies reported in the literature so far.
 Camelia Echim | Camelia Echim obtained the degree of Engineer in Food Sciences and Technology in 2004, a Master’s degree in Food Quality Management in 2006 and a doctoral degree in Biotechnology in 2008, at University of Agriculture and Veterinary Medicine Cluj-Napoca, Romania. Since 2007, she is doing her second PhD at the Faculty of Bioscience Engineering, University of Ghent, Belgium. She has published 6 scientific papers and her research interest is focussed on the use of bio-renewable resources as a source of biofuels and the determination of biodiesel quality. |
 Roland Verhé | Prof. Roland Verhé is Director of the Department of Organic Chemistry, Ghent University. He obtained his PhD at Ghent University in 1972. His research interests involve organic synthesis and the chemistry and technology of lipids for food and non-food applications. Research topics are dealing with determination of minor compounds in oils, the quality of speciality oils and the use of various lipid resources for biodiesel and biofuels. He is the coordinator of various international educational programs in the field of food sciences and renewable resources. |
 Wim De Greyt | Wim De Greyt graduated in 1988 as an Engineer in Food Technology from the Faculty of Applied Biological Sciences at the University of Ghent, Belgium. He obtained a PhD degree at the same University in 1998 with a study on the ‘Effect of Physical Refining on Selected Minor Components in Vegetable Oils’. Today, he is managing the Desmet-Ballestra R&D Centre located in Zaventem, Belgium. In the R&D centre, a group of 15 specialized R&D people are working on different R&D projects in the area of oilseed extraction, oil refining and modification and biodiesel production. He is (co-)author of more than 30 scientific papers. |
 Christian Stevens | Christian Stevens is currently full professor at the Department of Organic Chemistry at the Faculty of Bioscience Engineering (Ghent University, Belgium). In 2000, he became associate professor in Ghent and full professor in 2008. C. Stevens has published over 130 international peer reviewed scientific papers and reviews. He holds several patents on the synthesis and applications of renewable resources and is editor of the Wiley book Series on Renewable Resources. His research interest is focussed on synthetic heterocyclic chemistry related to agrochemical and medicinal applications and on the chemical modification of renewable resources. |
Broader contextIn the transition from a fossil fuel based to a bio-based economy, it is of crucial importance that the biological feedstocks (mainly plant material) will be valorized in an integral way. Too many times in the past, the biological material was used for a very low value application or discarded as waste after the valorization of one component. Also the synergy between food, feed and fuel production is a crucial aspect. This review discusses the possibilities to valorize the different side-streams of oil refining (from food and fuel production). After refining the oil, several important side-streams can be characterized and these can be transformed into biodiesel using different methods. The valorisation of the side-streams helps to reduce the waste, to minimize the footprint of the technology and to add value through the production of biodiesel as an energy carrier. |
1. Introduction
Refined vegetable oils are the predominant feedstocks used for the production of first generation biodiesel. However, their use for this application has led to an increased competition with food supply and oleochemicals. Alternative resources such as side-streams of refining (SSRPs) can partially replace the traditional feedstocks for the production of biodiesel, but require application of new technologies and/or additional purification steps. An overview of the typical composition, food and non-food applications and different processes used to convert the SSRPs into biodiesel/biofuel is given.1.1. Side-stream refining products and their typical composition
Crude vegetable oils contain triacylglycerols (TAG) as a major component and various minor components such as diacylglycerols (DAG), monoacylglycerols (MAG), free fatty acids (FFA), phospholipids, tocopherols, sterols, squalene, color pigments, waxes, aldehydes, ketones, triterpene alcohols and metals that may affect the quality of the final product. The minor components are removed partially or entirely by either physical (RBD) or chemical refining (NBD) in order to make the vegetable oils suitable for human consumption. Chemical and physical refining routes differ in procedures to remove free fatty acids, which is done by chemical neutralization (NBD) or by distillation (RBD), respectively.The first step of both refining processes is degumming, designed to remove the solid phospholipids (gums) also known as oil sediments (OS). Upon chemical refining, a weak alkali solution is added to neutralize free fatty acids which are washed out of the oil as soaps. This wet lipid mixture is termed soapstock (SS).
In the bleaching process coloring pigments, residual phosphatides, soaps and metals are removed by means of acid activated bleaching earth. The generated solid waste is termed spent bleaching earth (SBE).
The last step of refining process is deodorization, aimed to remove odoriferous components and free fatty acids from the oil by vacuum steam distillation generating a side-product termed deodorizer distillate (DD).
Physical refining is mainly used for high free fatty acid (FFA) feedstocks such as palm oil, but also on low FFA oils. Palm oil is generally refined by the physical process, which is preferred over the chemical process since high acidity (up to 5%) can lead to excessive loss of neutral oil in the soapstock after alkali neutralization.1
The composition of side-stream refining products has been well documented by different authors (Table 1).2–5
| Compounds (%) | Soapstock | Acid oil | Deodorizer distillates | Spent bleaching earth | |
|---|---|---|---|---|---|
| RBD a | NBD b | ||||
| a RBD = physical refined (refined, bleached and deodorized). b NBD = chemical refined (neutralised, bleached and deodorized). | |||||
| Water | 32–67 | <1–3 | — | — | — |
| Free fatty acids | 10–28 | 39–79 | 80–90 | 30–60 | 11–13 |
| Acylglycerols | 12–13 | 18–30 | <1–14 | 5–12 | up to 30 |
| Phospholipids | 5–9 | — | — | — | traces |
| Unsaponifiable matter | <1 | <1–4 | 5–10 | 25–33 | traces |
It was seen that the composition of DD is dependent on the oil source, the refining routes and the deodorizer operating conditions.6,7DD obtained from physical (RBD) and chemical refining (NBD) of different feedstocks contain typically 33–81% FFA, an important unsaponifiable matter such as tocopherols, sterols and squalene (6.6–41.2%), but also acylglycerols (0.72–13.6%).3
SS has a semisolid consistency and physico-chemical properties changing with the type of vegetable oil source, seed processing, handling and storage conditions. SS consists of a heavy alkaline aqueous emulsion of lipids (pH values between 10–11) mainly composed of water (32–67%),8 with the balance made up of FFA present as soaps (10–28%), phosphatides (5–9%) and acylglycerols (12–13%).2,31 Additionally, SS may contain other components depending on the starting feedstock's composition, such as xanthophylls,9gossypol and carbohydrates (sucrose, raffinose, stachyose).8
SS is normally acidulated by adding mineral acid to liberate free fatty acids and aqueous saline phase. The process generates a fraction which is generally dark in colour known as acid oil (AO) or acidulated soapstock (ASS) that contains a low amount of water (0.8–3.1%),10 protonated free fatty acids (39–79%), acylglycerols (18–30%) and unsaponifiable matter (0.4–4.2%).11
SBE contains a high percentage of FFA (11.5–12.6%), phosphorus (15.8–19.3 ppm), iron (0.22–1.24 ppm), copper (0.32–0.38 ppm), β-carotene (3–7 ppm) and other compounds.12
1.2. General applications of side-stream refining products (SSRPs) and estimates of their production
The SSRPs have found multiple applications due to their heterogeneous composition.SS and AO are normally used as 1) a source of free fatty acids, 2) low-grade oil for industrial applications, 3) medium grade soap products, 4) low cost oleochemicals for petroleum and rubber industries,13 5) top coating to reduce dust on gravel roads but also 6) components of animal feeds. It was found that SS and AO could be successfully used in broiler rations as an energy source instead of crude vegetable oils.14 Studies showed that acid oil can be safely included in the broiler diet up to 3% without affecting production performance with an increase in net profit per kg live weight.15 However, there may be some inherent problems associated with their use due to residual sulfuric acid and its reaction products (sulfates and sulfonates), which decreases palatability in most animals.
DDs represent a good source of valuable compounds such as phytosterols, tocopherols and squalene, which can be recovered and further used as food additives, in the pharmaceutical industry and cosmetics. Furthermore, the free fatty acids from DD are mostly used as additives for animal food, fluidizing agents for lecithin or as medium-grade soaps. Such fatty acids also can be used as precursors in a wide variety of molecular synthesis schemes such as the production of dibasic acids of different chain lengths.16 Alternatively, DDs have nonfood applications, such as being mixed with the fuel oil to fire the steam boilers (5% to 10%).17
SBE was disposed off by incineration, inclusion in animal feeds, land filling or concrete manufacturing.
The conversion of SS,2,10,29,39,41,42,44 AO,30,32–38,40,43,45DD46–59 and SBE12,18 to biodiesel/biofuel have been studied by different authors and a summary of the existing processes is given in this review.
Rough estimates of the quantity of SSRP are available; the amount mainly depends on the content of FFA, gums and impurities present in the oil and on the efficiency of refining. Using the Malaysian figures for the worldwide early production of crude vegetable oils in 200719 and assuming that 1) palm oil is entirely physically refined (100% RBD), 2) soybean and rapeseed oil are partially physically and chemically refined (50% RBD/50% NBD) and 3) sunflower oil is mainly physically refined (75% RBD/25% NBD), one can estimate the deodorizer distillate and soapstock production. Considering that 1) by physical refining a multiple of 1.2 times the FFA content are removed from crude oil as DD, 2) by chemical refining a multiple of 1.7 the FFA content are removed from crude oil as SS (dried product) or AO,20 and that 3) SS (wet product) contains in average 50% more water, the SSRP production can be estimated (Table 2).
| Oil crop | Crude Oil productiona (million t/year) | FFA (%) | Side stream refining products (mil. t/year) | ||
|---|---|---|---|---|---|
| DD b | SSd | AOc | |||
| a Source: Malaysian Palm Oil.19 b DD = 1.2 × FFA of crude oil.20 c AO = 1.7 × FFA of crude oil.20 d SS = 2 × AO. | |||||
| Palm | 36.84 | 4.0–5.0 | 1.77–1.84 | — | — |
| Soybean | 35.26 | 0.5–1.0 | 0.11–0.21 | 0.30–0.60 | 0.15–0.30 |
| Rapeseed | 18.36 | 0.5–1.0 | 0.06–0.12 | 0.16–0.32 | 0.08–0.16 |
| Sunflower | 11.10 | 2.0–3.0 | 0.20–0.30 | 0.18–0.28 | 0.09–0.14 |
| Total | 101.56 | 7.0–10.0 | 2.14–2.47 | 0.64–1.20 | 0.32–0.60 |
Considering these estimates, the total production of SSRPs is relatively low to replace the vegetable oils for the production of biodiesel. The SSRPs can eventually represent one of the alternative feedstocks together with the new generation feedstocks (animal fats, cooking oils, jatropha and algae oil).
2. Production of biodiesel from side-stream refining products (SSRPs)
2.1. Introduction
The most common method to produce biodiesel from vegetable oils with a low FFA content is the transesterification using homogeneous base catalysts (e.g., NaOH, KOH, NaOCH3, KOCH3). For feedstocks with high FFA content, the most common method to produce biodiesel is esterification using homogeneous acid catalysts (e.g., H2SO4, KHSO4, p-toluene-sulfonic acid or methane sulfonic acid). Both acid and basic homogeneous catalyzed processes require downstream purification equipment to neutralize the catalyst and to purify the biodiesel, which increases the production cost. The cheapest and the best known homogeneous acid catalyst used for the esterification reaction is H2SO4. The main disadvantages of this catalyst is the possibility that at the high temperature the more unsaturated fatty acids would undergo polymerization and that side products like dark color oxidized or other decomposition products could be formed. Furthermore, the use of strong acids implies the need for special equipment (e.g., stainless steel) and care for handling.A survey of the literature revealed that a wide variety of heterogeneous base and acid catalysts are under investigation. The most tested feedstock is waste cooking oil or synthetic acidic feedstocks, and less the side stream refining products. The most frequently cited heterogeneous catalysts are the strongly acidic sulfonated ion exchange resins,21,22 zeolites,22 mixed metal oxide,23,37 mesostructured silicas24 and mesoporous carbon.25
The use of biocatalysts (lipases) has also been reported.2,39,40,43 One of the main disadvantages associated with their use, is the high price compared to chemical catalyst.
A non-catalytic biodiesel production route with supercritical methanol has also been developed.26 Unlike the alkali-catalyzed method, the presence of water positively affected the formation of methyl esters. However, the high excess of methanol, which has to be used in supercritical conditions, makes the process economically unfavorable. The use of lower amount of methanol has been reported for two step process, where acylglycerols were hydrolyzed to FFA and further esterified to FAME.27
2.2. Production of biodiesel from soapstock (SS) and acid oil (AO)
The production of biodiesel from AO encounters less difficulty due to the lower water content compared to the SS. This implies lower cost for handling. However, AO, like SS, may contain other impurities (e.g., phospholipids). The later acts as an emulsifier during washing step or leads to an increased difficulty in the phase separation between FAME and glycerin. It was reported that the pre-treatment of the AO by filtration and warm centrifugation (80 °C) can reduce the amount of phosphorus compounds and has a direct positive impact on the quality and yield of the final esters.30
There is a general trend to pre-treat the SS before converting it to FAME, either by 1) acidulation, in order to generate AO (soap-splitting route)10,32–38 or 2) by hydrolysis of neutral oil (hydrolysis route).28,41–44 However, some authors reported the 3) direct conversion of SS to FAME after drying, filtration or transesterification2,31 or 4) esterification with glycerol to acylglycerols prior transesterification.30,45
Direct conversion of SS into biodiesel is achieved on the lab-scale. Industrially, it is feasible only via soap-splitting route.
Different routes to convert SS to biodiesel are represented in Fig. 1.
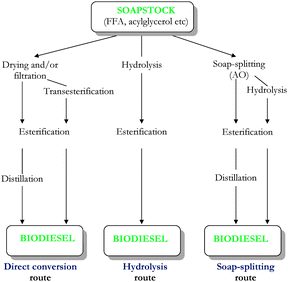 | ||
| Fig. 1 Schematic representation of different routes used to convert soapstock to biodiesel. | ||
Keskin et al.31 described a process where the splitting of the soaps and esterification was performed in one step. The cotton oil SS containing 85.3% FFA was filtered and dried for removing water and impurities before esterification in the presence of H2SO4, for 8 h followed by the separation of glycerol, water, catalyst and washing of the crude biodiesel with sodium bicarbonate, which was further distilled to obtain pure biodiesel.
The author studied the use of blends of cotton oil SS biodiesel and diesel fuel in a diesel engine. It was showed that the power output and torque of engine decreased by 6.2% and 5.8%, respectively. Specific fuel consumption value with blend fuels increased by 10.5%, depending on the amount of biodiesel used for blending and engine speed. The lowest percent heat losses to exhaust were obtained with blend of B20. However, percent heat losses to exhaust of B40 and B60 were similar with values of diesel fuel.
Particulate material emission of the engine with blend fuels at maximum torque speed decreased by 46.6%.
a. Chemically catalyzed process. Eaves et al.32 developed a method for converting AO to methyl esters using HCl or Twitchell reagent. Dehydration of the material and restoration of the initial methanol to fatty acid ratio increased the yield of esterified fatty acids by 5%. Pretreatments such as hydrolysis of the AO prior esterification, in order to decrease the content of neutral oil to less than 1%, passing the material through the reactor for the second time with a restoration of the initial methanol to fatty acid ratio improved the ester yield.
Basu and Norris33 described a process for producing methyl esters from AO containing 52% FFA. The catalyst (0.5%) used for the reaction was a powder mixture of calcium acetate and barium acetate in a ratio of about 3 : 1, which had the advantage that it did not catalyze the formation of soaps consequently allowing the elimination of a pretreatment step. After methanol removal and further purification of methyl esters a yield of 81.7% crude methyl esters was obtained containing still residual FFA (∼4%) and MAG (∼5%). Although a distillation step of the crude methyl esters reduced the FFA content to 2.2%, the quality of biodiesel was still not complying with the specifications making necessary the application of an additional esterification with sulfuric acid and methanol in order to reduce the FFA content to below 0.5%.
Haas et al.34 described a method for the production of methyl esters using soybean AO which contained 59.3% FFA, 28.0% TAG, 4.4% DAG and less than 1% MAG. Maximum esterification occurred at 65 °C after 26 h reaction but the reaction did not proceed to completion: more than 15% of the FFA remained in the mixture in free or glycerol-linked form (acylglycerols). In an alternative approach, the acylglycerol species in SS were saponified by the injection of steam at pH values >11 prior to acidulation to a final FFA content of 96.2% and no detectable TAG, DAG or MAG. After the esterification reaction the product did not contain any detectable acylglycerols but the FFA were still unreacted. It was postulated that the accumulation of water released by esterification had prevented complete esterification. The second esterification reaction step, following removal of this water by centrifugation, reduced the content of unreacted FFA to an acceptable level set by the specification for biodiesel.
Luxem and Troy35 described a one step process for converting both FFA and acylglycerols to alkyl esters, at temperatures between 130 °C and 150 °C under pressure up to 3.45 MPa. It was seen that acylglycerols were not completely transesterified at atmospheric pressure, although the acid value was reduced to 0.5 (mg KOH/g) within 6 h, which made necessary the use of higher pressure. Although the removal of the by-products formed during reaction (water and glycerol) and the addition of fresh methanol and make-up catalyst improved the conversion rate of FFA, the addition of a dehydrating agent (anhydrous sodium sulfate) was necessary to reduce the acid value to 0.5.
Geier et al.36 described a method, which uses simulated moving bed chromatography in the presence of acidic cation exchange resin to convert the canola oil (∼40% FFA) and soy/coconut AO (∼30% FFA) into fatty acid alkyl esters. A pre-treatment of the feed material consisted of distillation or contact with a strong acid cation resin and/or contact with an acid.
Different resins were tested for conversion of fatty acids to fatty acid methyl esters in the presence of methanol, at 60 °C using 50 ml columns. The maximum conversion of 95% was achieved. It was shown that by applying an acidified methanol (0.1% HCl) to the ion exchange pre-treated feedstock, having installed Lewatit K2629 Sybron resin in the Reactive Simulated Moving Bed Chromatography (RSMB) unit, the resin life was prolonged and the conversion was increased to 99.5% after 2 runs and by the third run the conversion increased to 99.9%.
Wang et al.10 described a process for biodiesel production from SS via AO production route. The author reported an improvement of the AO recovery from SS by using deionized water and sulfuric acid under the ambient temperature, followed by the conversion of AO into biodiesel in a pressurized reactor.
AO containing 50.0% FFA, 25.5% acylglycerols and other inert materials (23.7%) was esterified to methyl esters using sulfuric acid as catalyst. It was seen that less methanol was necessary if the water content of the starting material (AO) was lower. The quality of the final biodiesel was increased to 97.6% by distillation which represented a final average yield of 94% based on the total fatty acids in SS.
McNeff et al.37 studied the use of metal oxide catalysts (zirconia, titania and alumina microspheres) for the conversion of a wide variety of feedstocks to methyl esters in a continuous fixed bed reactor under high pressure and elevated temperature (between 300 °C and 450 °C) without loss of catalytic activity over extended use (Mcgyan process). The catalysts showed a good conversion of both AO and refined triglycerides sources to biodiesel. The unreacted free fatty acids were recovered by adsorption onto an alumina packed-bed polisher and further used together with residual biodiesel and alcohol in the Mcgyan process.
Recently Jin et al.38 described a process in three steps for producing FAME and phosphatides from a mixture of OS and SS.
The OS–SS mixture was extracted with ethyl ether and the mixture was separated into three phases by centrifugation after addition of saturated NaCl solution in order to minimize the losses of TAG and phosphatides. The soap phase was acidified with sulfuric acid to yield FFA and the resulting AO was converted to methyl esters by acid catalyzed esterification (second step). FAME recovery under these conditions was 92.1%. The organic top phase was treated with acetone for separation of phosphatides and triacylglycerols and further transesterified with NaOH, for 1 h at 65 °C, yielding 94% methyl esters.
b. Enzymatically catalyzed process. Sato et al.39 described a process for production of fatty acid esters from SS containing 40% water, where the lipid fraction contained 95.2% FFA as soaps, 1.0% of sterols and 3.8% partial glycerides. The soaps were neutralized and split with strong acids until reaching pH ∼ 4. This step was followed by enzymatic esterification with was conducted without prior separation of the aqueous phase. Ester phase was separated by settling out and pumped to a centrifuge, or the solid fraction was filtered out in order to facilitate the separation. Crude esters were distilled using a batch technique or continuous flash distillators. A residual amount of water or methanol was stripped off using degasser prior to main distillation. The distillation pitch was processed to recover the sterols and fatty acids or recycled back to the SS storage tank. Esterification yield between 40%–42% was reported to the starting SS, and reached 71%–74% if considered to the total fatty material.
Watanabe et al.40 described a two step process comprising esterification of FFA and methanolysis of acylglycerols to FAME using AO model containing 78% FFA, 10% acylglycerols, 3% sterols and 8% of other unknown lipophilic compounds. Esterification was conducted using immobilized Candida antarctica lipase. The first step yielded 77% FAME (96% conversion of the FFA). The second-step reaction determined an increase of FAME content to 91% with the decrease of acylglycerols and FFA content from 9.7% to 0.9% and from 2% to 0.8%, respectively. However, the reaction was very slowly, 24 h being necessary for each step.
a. Chemically catalyzed process. A process was developed to obtain biodiesel from soybean SS which met the specifications for all assayed parameters.29,41 First, an alkali-catalyzed hydrolysis step (saponification) of the lipids was adopted, converting all acylglycerols to free fatty acids. This step was followed by esterification of the resulting free fatty acid product. The most rapid route to achieve a complete saponification of both acylglycerols and phosphoacylglycerol involved the addition of sodium hydroxide to a final concentration of 4.2% followed by incubation at 100 °C for 2 h to 4 h. Water formed during the reaction was removed by lyophilization, the resulting dried saponified SS which contained a significant amount of sodium hydroxide were converted to fatty acids methyl esters by incubation with methanol and sulfuric acid at a reaction temperature of 35 °C and ambient pressure. It was observed that esterification took place even if residual water up to a level of 10% was present in the saponified SS reactant. In order to purify the crude biodiesel from traces of alcohol, acid, glycerol and small amounts of unreacted FFA, the reaction product was successively washed with aqueous solutions of NaCl, NaHCO3 and CaO. The crude biodiesel contained more than 99% FAME, not detected TAG, <0.05% DAG, <0.1% MAG, and <0.8% FFA. The FFA content was further reduced by washing with diluted sodium hydroxide. Although the process generated a high quality biodiesel, the yield was 60% of the theoretical amount of product of FAME, the remaining methyl esters being located in the solid fraction.
A process to yield alkyl esters from SS having a moisture content of 65% has been described.42 The technology consists of 1) saponification of the acylglycerols present in the SS with an alkaline base (NaOH) and alcohol (ethanol or methanol), at the temperature between 65 °C and 90 °C at atmospheric pressure, and 2) filtration to remove substantially alcoholic insoluble organic material (gums and lecithin). The following step consisted in 3) splitting of the soaps with an excess of mineral acid (H2SO4) under the same reflux conditions to form fatty acids and a mineral salt, and 4) esterification of the fatty acids with alcohol and acid already being used. Each step was conducted at the boiling point of the solvent, for 30–90 min. After neutralization of the acid and removal of the generated salts, the excess alcohol was recovered by distillation followed by the removal of insoluble materials and distillation of the alkyl esters.
The removal of the by-products generated during the entire process allowed to increase the quality of the final biodiesel and to recover other organic and inorganic compounds. This process resulted in a biodiesel yield of about 32% reported to the starting soapstock or about 93% reported to the organic phase.
b. Enzymatically catalyzed process. Watanabe et al.43 described a method for converting AO to fatty acid methyl esters comprising hydrolysis of neutral oil to FFA followed by two steps esterification. Acylglycerols were enzymatically hydrolyzed. The resulting layer containing 92% FFA was used for the methyl esterification to FAME by immobilized Candida antarctica lipase. The degree of esterification reached 96% after 24 h. The resulting reaction mixture was then dehydrated and subjected to the second esterification step. The degree of esterification of residual FFA reached 44%. Furthermore, the degree of esterification increased successfully to 72% by conducting the reaction in the presence of 10% glycerol, which acted as an in situ dehydration agent at ambient pressure. Over 98% of the total esterification yield was maintained, even though the first and the second esterification reactions were repeated every 24 h for 40 days. Although a two step esterification process was applied, the resulting methyl esters still contained unreacted material (1.1% FFA and 0.9% acylglycerols).
A two step process to produce fatty acid alkyl esters via enzymatic catalysis, using SS waste as a feedstock, was described by Kempers et al.44 First, a lipase was added to facilitate hydrolysis of acylglycerols and phospholipids before or after neutralization, and then the soaps were split with strong acids until they reached a pH between 4 and 6. As a final step, the fatty acid phase was separated from the aqueous phase by settling and/or by centrifugation and was further purified by distillation. The resulting FFA were enzymatically esterified to the corresponding alkyl esters. The achieved conversion was between 60% and 95%. In order to achieve a good separation of esters from residual FFAs, the later were turned into their ionic form after enzymatic esterification by addition of a mineral base like KOH, NaOH or Ca(OH)2 solution to prevent co-distillation with the alkyl esters. Furthermore, the sterol esters were enriched in the residue from the distillate, which could be further purified by crystallization, extraction or fractional distillation, and to be used for other applications.
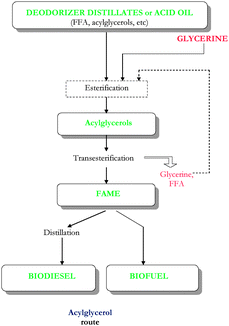 | ||
| Fig. 2 Production of biodiesel/biofuel via acylglycerols route from high acidity feedstocks (deodorizer distillates or acid oils). | ||
a. Chemically catalyzed process. Luxem and Mirous30 compared the activity of different acid, base and transition metal catalysts. The reactions have been performed using AO and crude (neutralized) glycerine at 180 °C, for 4 h. The amount of catalyst was normalized based on the equal ratio of metal content per mol of acid. The results showed that the organo-metal catalysts (tetrabutyltitanate, dibutyltin oxide and tin oxalate) were the most efficient catalysts. Brønsted acids were less effective, the formation of acrolein being observed. The highest conversion (93%) was achieved using 1% tin oxalate, whereas DBTO achieved 81% conversion using 0.2% loading.
The process has been scaled-up using dibutyl tin oxide as catalyst and longer cycle times, resulting in acylglycerols with an acidity of 0.5 (mg KOH/g). The esterification product was directly converted via base catalysis into biodiesel (95%) which was subsequently distilled to give ASTM specification complying product, with a final yield of 92%.
b. Non-catalytic process. Luxem et al.45 described a two-step process involving first the non-catalytic conversion of the FFA of AO into a mixture of MAG, DAG and TAG, and subsequently transesterification of the acylglycerols to biodiesel. The method for making biodiesel was tested both on a small scale (1 L) and on a larger scale. The process implied an addition of glycerin, at 180 °C and reduced pressure. It was found that crude glycerin derived from methanolysis of vegetable oils or other fats, or recovered from a fat splitting process, was more effective than higher grade glycerin. If crude glycerin was used for the esterification of FFA, a shorter reaction time (∼10 h) was necessary compared with the case when USP grade glycerin was used (∼14 h). If a combination of vacuum and nitrogen was used for removing the water formed during reaction, the overall reaction time to reach a comparable acid value was significantly reduced (from 14 h to 10 h).
The resulting mixture was subjected to a base catalyzed alcoholysis, for 1 h to convert the newly created glycerides, as well as the originally present glycerides, into fatty acid alkyl esters for use as biodiesel. The glycerin generated in this second step of the process was recycled and used together with crude glycerol from the methanolysis of soybean oil (20% and 80%, respectively) as starting materials for synthesis of acylglycerols. The crude biodiesel was subjected to vacuum distillation after glycerol separation and washing, yielding between 85% and 90% colorless methyl esters.
2.3. Production of biodiesel from deodorizer distillates (DD)
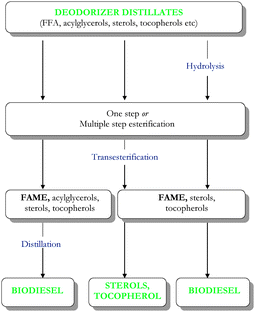 | ||
| Fig. 3 Production of biodiesel, sterols and tocopherols from deodorizer distillates. | ||
a. Chemically catalyzed process. Soragna46 described the industrial process for the conversion of FFA into FAME, using heterogeneous catalyst, called FACT (Fatty AcidConversion Technology). This technology is an alternative option compared with the classical technology using homogeneous catalyst, consisting of the continuous countercurrent multiple step esterification using solid catalyst in the fixed bed reactors, at 90 °C and 0.35 MPa. Production of biodiesel/biofuel from feedstocks with high acidity by direct conversion was registered as a “stand alone process” (Fig. 4, a).
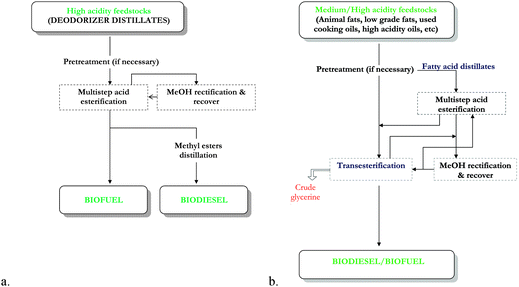 | ||
| Fig. 4 Fatty Acids Conversion Technology (FACT) to produce biodiesel/biofuel from low quality raw materials. a. Stand alone process. b. Integrated process. From Soragna.46 | ||
For feedstocks with medium/high acidity, an “integrated process” was applied (Fig. 4, b) where a transesterification step for the conversion of the acylglycerols was also included. The FFA were distilled off and further esterified to FAME before the transesterification of the residual acylglycerols.
The advantage of these processes is the possibility to process high diversity acidity feedstocks (up to 100%) with a conversion up to 99.8% without limitation in capacity, no usage of liquid acids, higher quality by-products and mild operating conditions.
Verhé et al.47 reported a process of converting the DD to biodiesel using sulfuric acid as catalyst, at 75 °C for 5 h. The FFA and MAG have undergone esterification, resulting in methyl esters. The crude biodiesel was further washed, dried and distilled in order to increase the quality of the methyl esters. The distillation pitch was further processed for the recovery of sterols and tocopherols.
An extensive study was done by Chongkhong et al.48 on the palm fatty acid distillate (PFAD) (93% FFA), as feedstock for a batch and continuous production of biodiesel. For the continuous process (CSTR), the amount of FFA was reduced from 93% to less than 2% at the end of the esterification process. A further treatment consisting of neutralization of the FFA and transesterification of the glycerides was required in order to obtain biodiesel, which complies with the specifications.
Facioli and Barrera-Arellano49 described a process to obtain ethyl esters from soybean oil deodorizer distillates (SODD) using concentrated H2SO4 as catalyst. The DD contained 47% FFA, 26% acylglycerols and 26% unsaponifiable matter.
A conversion of 94% of the fatty acids to ethyl esters was achieved. However, the acylglycerols were not affected and the losses of tocopherols were around 5.5%. A molar excess of ethanol in relation to SODD:FFA was found to be necessary to obtain the best conversion.
Hammond and Tong50 described a three-stage acid catalyzed esterification. The reaction mixture was centrifuged, the supernatant lipid phase was separated from the sludge (glycerol, water, acid and methanol), and further reacted with methanol and acid. The maximum FAME conversion obtained for 12 tested acid oils averaged 81%. However, the ester phase could not be increased above 85% even after a fourth-stage reaction or if a base catalyst was used in large excess. Unknown materials were reported in both FAME and sludge phase having a hydrophobic and hydrophilic behavior, respectively. The former compound determined an increase of the biodiesel viscosity and is hypothetically attributed to the presence of polymers. The polymers might have been formed during the soap acidulation process or during the esterification reaction, due to the limited supply of methanol substrate and the long reaction time. The compound could not be further removed by distillation, which was explained by the fact that vapor pressure was not low enough to allow a good separation from FAME.
b. Enzymatically catalyzed process. The lipase-catalyzed methyl esterification of the fatty acids present in canola oil deodorizer distillates (CODD) was studied by Ramamurthi et al.51
CODD was esterified to methyl esters, using immobilized lipaseRandozyme SP-382 as catalyst. Conversion of FFA up to 96.5% was achieved without the use of vacuum or dehydrating agent.
It was found that three variables—namely moisture content of the enzyme, reaction time and the amount of a molecular sieve—did not exhibit any profound effect on the conversion rate. On the contrary, the ratio of the reactants had a significant effect on the conversion equilibrium and showed a high interaction effect along with temperature. High conversion (>90%) was obtained at combinations of both high temperature (70 °C) and a low ratio of reactants (1.2) and for combinations of low temperature (50 °C) and a high ratio of reactants (2.0). It was seen that higher concentrations of enzymes could compensate the negative effect of increased temperature. The conversion of acylglycerols was not investigated in this study, since the esterification was considered as a preliminary step to the recovery of tocopherols and sterols.
Facioli and Barrera-Arellano52 investigated the enzymatic esterification of the FFA from SODD with ethanol using immobilized fungal lipase (LipozymeIM) as biocatalyst. SODD contained 47% FFA, 26% neutral oil and 26% unsaponifiable matter. The best conversion was above 88% with no tocopherol losses.
The esterification of SODD with butanol, using Mucor miehei lipase as the biocatalyst and supercritical carbon dioxide (SC-CO2), has been described by Nagesha et al.53 The SODD contained 56% neutral oil, 25% FFA, 7% sterols, 3% tocopherols, <1% hydrocarbons and 0.1% moisture. The feedstock was preliminarily filtered in order to remove sediments and sterols and enzymatically hydrolyzed to free fatty acids using immobilized lipase (Candida rugosa) in a SC-CO2 reactor unit. Hydrolyzed SODD containing <88% FFA was enzymatically esterified further with M. miehei in the presence of butanol, with a maximum yield of 95% FABE. The content of acylglycerols was not affected by esterification. The high content of residual glycerides (3%) present in the final FABE impeded its direct use as a biodiesel. However, the process was designed as a preliminary step for the purification of tocopherols, since hydrolysis/esterification is facilitating their recovery.
Wang et al.54 described a process for simultaneous conversion of FFA (28%) and acylglycerols (60%) from SODD to alkyl esters using a mixture of two enzymes (3% Lipozyme TL IM and 2% Novozym 435) in the presence of tert-butanol as a co-solvent. It was found that the negative effects on the enzyme stability caused by the excessive methanol ratio and by-product glycerol could be completely eliminated by using tert-butanol. The lipase activity remained stable after 120 cycles. The maximum yield of FAME (84%) was achieved with an increase of tert-butanol content up to 80% (based on the oil weight). However, a further increase of the solvent resulted in a decrease of the ME yield which was explained by the dilution effect on reactants.
Fine-porous silica gel and molecular sieves (3 Å) were found to be effective to improve biodiesel yield by controlling water concentration formed as a by-product during reaction. A conversion yield of 97% could be achieved when 3 Å molecular sieves was used as adsorbent (10 times maximal water weight). The use of lower amount of silica gel gave similar conversion rates (93%). However, more than 10 times silica gel lead to a decrease in the ME yield, which was explained by the reduced availability of methanol for the methanolysis due to its absorbance by the drying agent.
Du et al.55 investigated the enzymatic esterification of SODD containing 28% FFA, 60% TAG and 6% tocopherols. The reaction was lipase-mediated methanolysis using Novozym 435 as catalyst, at 40 °C in a solvent free medium. The enzyme kept its activity after being reused for 10 cycles, each cycle of 24 h. The highest biodiesel yield of 95% was achieved by adding 10 fold molecular sieves (3 Å). The investigation of the lipase to methanol tolerance revealed that the lipase could maintain its stability and activity in the presence of methanol at even 3 molar. This tolerance was attributed to the presence of other compounds than TAG, namely FFA, sterols and tocopherols. A linear relationship between the FFA content and the lipase tolerance to methanol was observed but the presence of sterols and tocopherols showed no effect.
Synthesis of MAG from DD was mainly studied due to the large number of applications as additives, for enhancing plasticity of fats or as bases in the food, medicine and cosmetic industry. Among synthesized acylglycerols, the monoester has the highest surface activity and therefore, its concentration is very important for direct usage of reaction product as an emulsifier.
The esterification of glycerol with fatty acids leads normally to a mixture of MAG, DAG, and TAG and some amount of unreacted substrates. The proportions depend on the presence and type of catalyst, as well as the reaction conditions such as temperature and the molar ratio FFA:glycerol.
Several chemical methods are available for the preparation of individual and stereochemically pure glycerides. Homogeneous catalysts both basic (e.g., NaOH, KOH) and acidic (e.g., p-toluene sulfonic acid) are commonly used in the direct esterification of glycerol.
Studies showed that enzymes have an enormous potential as catalysts in the processes where high regioselectivity is required.56 However, for the large-scale synthesis, the processes are not yet competitive due to the high cost of the enzyme.
Although the use of a large number of different heterogeneous catalysts have been reported in literature, most of the research has been done on the synthetic samples and less on the SSRP.
Different studies summarized hereunder describe processes for synthesis of acylglycerols in order to decrease the acidity of the feedstocks. These processes are catalyzed either enzymatically, or conducted under non-catalytic conditions.
a. Enzymatically catalyzed process. Lo et al.56 reported a process to synthesize acylglycerols (mainly DAG) by lipase catalyzed esterification of glycerol with fatty acids from corn oil deodorizer distillate (CoDD). It was found that Lipozyme RM IM was the most effective among the commercial 1,3-position specific screened lipases. A DAG yield of 70% was obtained.
Tangkam et al.57 described the enzymatic esterification in a solvent free medium of different DD resulting from the refining of various vegetable oils. A direct esterification of mixed distillates (61% FFA and 39% acylglycerols) with glycerol using immobilized lipase B from Candida antarctica (Novozym 435) led to moderate proportions (46%) of DAG. Application of a two-stage reaction consisting of a hydrolysis step of DD to increase the FFA content followed by esterification with glycerol led to a higher formation (>61%) of DAG. Furthermore, it was observed that the high initial concentration of unesterified fatty acids in the distillate (100% FFA) has a positive influence on the concentration of DAG in the final product (>71%). Enrichment of DAG in the final products by short-path vacuum distillation led to concentrates containing up to 94% DAG, ∼5% TAG and no unesterified fatty acids and MAG. Increase in temperature strongly affected the rate of esterification, whereas the influence of the reaction pressure was only moderate.
b. Non-catalytic process. Smet59 described a process for the esterification of fatty acid distillate (93% FFA) with technical grade glycerol. The novelty of the process consists in synthesizing acylglycerols in a relatively short time (<6 h) without the use of catalysts obtaining a final yield of 86%.
However, the FFA content was still high, a distillation step of the residual FFAs and glycerol was necessary in order to increase the purity of the synthesized acylglycerols. The by-products of distillation were further re-used as reaction products in the synthesis of acylglycerols.
3. Alternative processes
Processes which were tested on feedstocks with a lower FFA content, but which may be applied for SSRPs, are summarized below.Kusdiana and Saka60 described a process performed in a two-step reaction, consisting of hydrolysis and methyl esterification. Hydrolysis was carried out at a subcritical state of water to obtain fatty acids from triglycerides of rapeseed oil, while the methyl esterification of the hydrolyzed products was treated near the supercritical methanol condition to achieve fatty acid methyl esters (FAME). A two-step reaction to obtain FAME in considerably shorter reaction time and milder reaction condition than the direct supercritical methanol treatment was found. The optimum reaction conditions for hydrolysis and methyl esterification were 270 °C for 20 min, respectively.
Iijima et al.61 proposed a new process called STING, based on the simultaneous reactions of transesterification and cracking under a supercritical condition. TAG, DAG, MAG and FAME, which consists of medium chain fatty acid, higher and lower alcohols, other hydrocarbons, are formed without the formation of by-products (e.g., glycerol) improving in this way the yield of the process. The resulting product has a lower viscosity and lower pour point compared to FAME formed by a conventional alkaline catalyzed process, mainly because part of the long chain fatty acids (C14 to C22) are decomposed into the short or middle chain fatty acids (mainly C6 to C10) by treating with supercritical methanol. These components form one phase and are used as a diesel fuel replacement.
Yamazaki et al.62 described a process called superheated methanol vapor bubble method, suitable both for refined and waste oils, and also crude oils rich in FFA, where superheated methanol vapor is blown into oil continuously and reacted with TAG and FFA to form fatty acid methyl esters and glycerol, using a reactor vessel at high temperature. The FAME and unreacted methanol vapor were collected by a condenser. It was observed that the reaction rate constant obtained with FFA was several times higher than that with TAG. It was estimated that the process applied in an industrial scale reactor could produce 1000 kg h−1FAME.
4. Conclusions
In the production of biodiesel/biofuel from SSRPs, their heterogeneous composition (FFA, acylglycerols, phospholipids, phospholipids, sterols and tocopherols) should be considered, not only for the selection of right conversion process but also for the valorisation of all the compounds which would make the process economically more interesting.There are multiple routes to converting the SSRPs to biodiesel/biofuel, some of which have found industrial application and others have a scientific value due to the big number of steps involved in the processing.
There is a general trend to pretreat the SS before converting it to FAME, either by acidulation, in order to generate AO (soap-splitting route) or by hydrolysis of neutral oil (hydrolysis route). Starting from DD two processes (direct conversion or via acylglycerols route) are shown to produce high quality esters (biodiesel). However, in both cases a pre-treatment of the feedstock or post-treatment of the final biodiesel is often required in order to meet the quality specifications.
Using a combination of technologies, low quality and price lipid resources can be converted into biodiesel that complies with the EU and ASTM specifications.
References
- V. Gibon, W. De Greyt and M. Kellens, Eur. J. Lipid Sci. Technol., 2007, 109, 315–335 CrossRef CAS.
- M. J. Haas and K. Scott, J. Am. Oil Chem. Soc., 1996, 73(11), 1393–1401 CrossRef CAS.
- T. Verleyen, R. Verhe, L. Garcia, K. Dewettinck, A. Huyghebaert and W. De Greyt, J. Chromatogr., A, 2001, 921, 277–285 CrossRef CAS.
- M. J. Dumont and S. S. Narine, Food Res. Int., 2007, 40, 957–974 CrossRef CAS.
- M. J. Dumont and S. S. Narine, Lipid Technology, 2008, 20(6), 136–138 CrossRef CAS.
- W. De Greyt and M. Kellens, in Edible Oil Processing, ed. W. Hamm and R. J. Hamilton, Sheffield Academic Press, Sheffield, 2000, 90–94 Search PubMed.
- M. Kellens and W. De Greyt, in Deodorization, Introduction to fats and oils technology, ed. R. D. O'Brien, W. E. Farr and P. J. Wan, Eds. AOCS Press, Champaign, 2ndedn., 2000, 235–268 Search PubMed.
- R. E. Beal and V. E. Sohns, J. Am. Oil Chem. Soc., 1972, 49(8), 447–450 CrossRef CAS.
- M. K. Down, J. Am. Oil Chem. Soc., 1996, 73(10), 1287–1295 CrossRef.
- Z. M. Wang, J. S. Lee, J. Y. Park, C. Z. Wu and Z. H. Yuan, Korean J. Chem. Eng., 2007, 24(6), 1027–1030 Search PubMed.
- S. Ghosh and D. K. Bhattacharya, J. Am. Oil Chem. Soc., 1995, 72(12), 1541–1544 CrossRef CAS.
- L. S. Kheang, C. S. Foon, C. Y. May and M. A. Ngan, Am. J. Appl. Sci., 2006, 3(10), 2063–2067 Search PubMed.
- N. O. V. Sonntag, J. Am. Oil Chem. Soc., 1985, 62(5), 928–933 CrossRef CAS.
- T. Balevi, B. Coskun and A. Aktümsek, Revue Méd. Vét., 2001, 152(11), 805–810 Search PubMed.
- N. S. Jayalakshmi, R. Mathivanan, R. Amutha, S. C. Edwin and K. Viswanathan, Int. J. Poultry Sci., 2006, 5(9), 890–894 Search PubMed.
- S. Gangopadhyay, S. Nandi and S. Ghosh, J. Oleo Sci., 2007, 56(1), 13–17 Search PubMed.
- C. Svensson, J. Am. Oil Chem. Soc., 1976, 53(6), 443–445 CrossRef.
- P. V. Lara and E. Y. Park, Enzyme Microb. Technol., 2004, 34(3–4), 270–277 CrossRef CAS.
- Malaysian Palm Oil, Fact sheets, in Malaysian Palm Oil Council and Malaysian Palm Oil Board, 2007, pp. 2–63 Search PubMed.
- R. J. Vries, J. Am. Oil Chem. Soc., 1984, 61(2), 404–407 CrossRef.
- M. M. R. Talukder, J. C. Wu, S. K. Lau, L. C. Cui, G. Shimin and A. Lim, Energy Fuels, 2009, 23(1), 1–4 CrossRef.
- A. A. Kiss, A. C. Dimian and G. Rothenberg, Adv. Synth. Catal., 2006, 348(1–2), 75–81 CrossRef.
- S. L. Yan, S. O. Salley, O. Steven and K. Y. S. Ng, Appl. Catal., A, 2009, 353(2), 203–212 CrossRef CAS.
- A. C. Carmo, L. K. C. de Souza, C. E. F. da Costa, E. Longo, J. R. Zamian and G. N. da Rocha, Fuel, 2009, 88(3), 461–468 CrossRef CAS.
- R. Liu, X. Q. Wang, X. Zhao and P. Feng, Carbon, 2008, 46(13), 1664–1669 CrossRef CAS.
- A. Demirbas, Biodiesel production from vegetable oils by supercritical methanol, J. Sci. Ind. Res., 2005, 64(11), 858–865 Search PubMed.
- S. Saka, D. Kusdiana and E. Minami, J. Sci. Ind. Res., 2006, 65(5), 420–425 Search PubMed.
- M. J. Haas, S. Bloomer and K. Scott, J. Am. Oil Chem. Soc., 2000, 77(4), 373–379 CrossRef CAS.
- J. B. Worfel, J. Am. Oil Chem. Soc., 1983, 60(2), 310–313 CrossRef.
- F. J. Luxem and B. K. Mirous, in Biocatalysis and Bioenergy, ed. C. T. Hou and J. F. Shaw, John Wiley & Sons, Inc., Hoboken, 2008, 115–129 Search PubMed.
- Keskin, M. Gürü, D. Altiparmak and K. Aydin, Renewable Energy, 2008, 33, 553–557 CrossRef.
- P. H. Eaves, J. J. Spadaro and E. A. Gastrock, J. Am. Oil Chem. Soc., 1959, 36, 230–234 CrossRef CAS.
- H. N. Basu and M. E. Norris, US Pat., 5 525 126, 1996.
- M. J. Haas, P. J. Michalski, S. Runyon, A. Nunez and K. M. Scott, J. Am. Oil Chem. Soc., 2003, 80(1), 97–102 CrossRef CAS.
- F. J. Luxem and W. M. Troy, US Pat., 6 768 015, 2004.
- D. F. Geier, A. K. Hilaly and J. G. Soper, US Pat., 0 245 405 A1, 2005.
- C. V. McNeff, L. C. McNeff, B. Yan, D. T. Nowlan, M. Rasmussen, A. E. Gyberg, B. J. Krohn, L. F. Ronald and T. R. Hoye, Appl. Catal., A, 2008, 343, 39–48 CrossRef CAS.
- B. Jin, M. Zhu, P. Fan and L. J. Yu, Fuel Process. Technol., 2008, 89, 77–82 CrossRef CAS.
- S. Sato, W. Bueno De Almeida and A. S. Araújo, WO Pat., 050 589, A1, 2006.
- Y. Watanabe, P. Pinsirodom, T. Nagao, A. Yamauchi, T. Kobayashi, Y. Nishida, Y. Takagi and Y. Shimada, J. Mol. Catal. B: Enzym., 2007, 44(3–4), 99–105 CrossRef CAS.
- M. J. Haas, S. Bloomer and K. Scott, US Pat., 6 399 800, 2002.
- R. Rohr and R. Rohr, US Pat., 0 225 341, 2006.
- Y. Watanabe, T. Nagao, Y. Nishida, Y. Takagi and Y. Shimada, J. Am. Oil Chem. Soc., 2007, 84(11), 1015–1021 CrossRef CAS.
- P. Kempers, U. Schoerken, T. Wolf, S. Sato, W. Bueno De Almeida, P. Silva Bizzari and A. S. Araujo, EU Pat., EP 1 876 222 A1, 2008.
- F. J. Luxem, J. H. Galante, W. M. Troy and R. J. Bernhardt, US Pat., 7 087 771, 2006.
- F. Soragna, 3rd Annual meeting, Berlin, 2008 Search PubMed.
- R. Verhé, V. Van Hoed, C. Echim, C. Stevens, W. De Greyt and M. Kellens, in Biocatalysis and Bioenergy, ed. C. T. Houand J. F. Shaw, John Wiley & Sons, Inc., Hoboken, 2008, 185–195 Search PubMed.
- S. Chongkhong, C. Tongurai, P. Chetpattananondh and C. Bunyakan, Biomass Bioenergy, 2007, 31(8), 563–568 CrossRef CAS.
- N. L. Facioli and D. Barrera-Arellano, Grasas y Aceites, 2002, 53(2), 206–212 Search PubMed.
- E. G. Hammond and W. Tong, US Pat., 6 965 044, 2005.
- S. Ramamurthi, R. P. Bhirud and A. R. McCurdy, J. Am. Oil Chem. Soc., 1991, 68(12), 970–975 CrossRef CAS.
- N. L. Facioli and D. Barrera-Arellano, J. Sci. Food Agric., 2001, 81, 1193–1198 CrossRef CAS.
- G. K. Nagesha, B. Manohar and K. U. Sankar, J. Supercrit. Fluids, 2004, 32, 137–145 CrossRef CAS.
- L. Wang, W. Du, D. Liu, L. Li and N. Dai, J. Mol. Catal. B: Enzym., 2006, 43, 29–32 CrossRef CAS.
- W. Du, L. Wang and D. Liu, Green Chem., 2007, 9, 173–176 RSC.
- S. K. Lo, B. S. Baharin, C. P. Tan and O. M. Lai, Food Biotechnol., 2004, 18(3), 265–278 Search PubMed.
- K. Tangkam, N. Weber and B. Wiege, Grasas y Aceites, 2008, 59(3), 245–253 Search PubMed.
- Y. Yamada, M. Shimizu, M. Sugiura and N. Yamada, WO Pat., 09 119, 1999.
- P. Smet, Karel de Grote-Hogeschool, Master thesis, 2008.
- D. Kusdiana and S. Saka, Appl. Biochem. Biotechnol., 2004, 115(1–3), 781–791 CrossRef.
- W. Iijima, Y. Hobayashi, K. Takekura, H. Kato and K. Taniwaki, 2007, APAN Field Server/Sensor Network Workshop, http://htttp://www.apan.net Search PubMed.
- R. Yamazaki, S. Iwamoto, H. Nabetani, K. Osakada, O. Miyawaki and Y. Sagara, Japan J. Food Eng., 2007, 8(1), 11–18 Search PubMed.
| This journal is © The Royal Society of Chemistry 2009 |
