A versatile new method for synthesis and deposition of doped, visible light-activated TiO2 thin films†
Su-il
In
a,
Alistair H.
Kean
b,
Alexander
Orlov
c,
Mintcho S.
Tikhov
d and
Richard M.
Lambert
*d
aCentre for Individual Nanoparticle Functionality, Department of Physics, Technical University of Denmark, DK-2800 Kgs, Lyngby, Denmark
bMantis Deposition Ltd, 2 Goodson Industrial Mews, Wellington Street, Thame, Oxon OX9 3BK, England
cMaterials Science & Engineering, State University of New York Stony Brook, Stony Brook, NY 11794-2275, USA
dDepartment of Chemistry, University of Cambridge, Cambridge, England. E-mail: rml1@cam.ac.uk; Fax: +44 1223 336362; Tel: +44 1223 336467
First published on 14th August 2009
Abstract
A flexible and widely applicable method allows the deposition of carbon-doped visible light-activated photocatalytic TiO2 thin films on a variety of substrates.
Broader contextVisible light-activated materials based on doped titania are the subject of intensive current research due to the promise they offer in relation to solar powered systems for photocatalysis, hydrogen production from water and hybrid systems for CO2 conversion. In most such applications, it is necessary for the photo-active phase to be present as a thin film. Current synthetic methodologies suffer from one or more serious shortcomings, which seriously hinder practical application. These include high cost, irreproducibility, difficulty in controlling the dopant level and unsuitability for scale up. We describe a new and versatile method that can reproducibly deposit films consisting of doped, size-selected TiO2nanoparticles with good control of thickness, particle size, and texture on a wide range of surfaces—metallic, insulating, rigid or flexible. It is well suited to scale-up and to the applications noted above for which visible light activation is critically important. In addition, the technique is readily adaptable to a variety of dopants and co-dopants. |
The global market for materials based on titania thin films is expanding rapidly, the best known large scale uses being photo-activated coatings for self-cleaning windows and buildings.1 Additional potential applications that are based on their photocatalytic activity,2 photoelectrochemical behaviour and photovoltaic response3 have also stimulated much basic research on these materials. Titania thin films may be produced by magnetron sputtering,4,5 sol–gel methods,6,7chemical vapour deposition and other techniques,8 but a major disadvantage of such materials is their complete lack of photoresponse under visible light irradiation: the 3.3 eV bandgap of anatase requires excitation wavelengths <376 nm. Given that less than 5% of the solar flux incident at the earth's surface lies in this spectral regime, harvesting sunlight for photocatalytic or photoelectrochemical purposes, including water splitting, would be enormously enhanced by extending the photoresponse of TiO2 into the visible region. This can be achieved by a low level doping with non-metals such as C,9N10 and/or B.11 Thus far, however, key issues including reproducibility and uniformity of doping are still far from resolved. Moreover, existing methods for producing doped TiO2 in thin film form are unsuited to large scale application and/or involve high energy routes.
Here we describe a new and versatile method that can reproducibly deposit films consisting of doped, size-selected TiO2nanoparticles with any desired thickness, particle size, and texture on a wide range of solid substrates. It is well suited to scale-up and to a wide variety of applications, for example self-sterilizing surgical instruments activated by ambient light. The present results refer to carbon doping: however the technique is readily adaptable to a wide variety of dopants and co-dopants, merely by changing the dopant precursor or mix of precursors (CH4, NH3, B12H10…). As will be shown, changing the level of carbon doping red shifts the optical absorption spectrum and endows the film with good photocatalytic activity.
Titanium nanoparticles were produced using a terminated gas condensation technique.12 A beam of negatively charged Ti nanoparticles was generated by high pressure DC magnetron sputtering in argon using a Ti target (Fig. 1). The nanoparticles produced under the conditions used had a diameter of ∼8 nm, estimated by quadrupole mass spectrometry that was carried out separately, and in accord with AFM observations on the as-deposited nanoparticle films†. This deposition technique generated thermalised nanoparticles, i.e. no heat was imparted to the surface on which deposition took place. Carbon doping was achieved by introducing variable amounts of methane into the Ar plasma by means of a precision leak valve. Direct measurement of the methane partial pressure was not possible as the argon partial pressure was several orders of magnitude higher. However, introduction of methane and its subsequent decomposition to carbon and hydrogen affected the magnetron target voltage which thereby provided a means of monitoring the stability of the gas composition during film deposition and gave an indication of the relative amounts of carbon being incorporated into the titanium nanoparticles. As shown below, XPS results provided a quantitative estimate of film carbon content. After exiting a second aperture into the deposition zone the nanoparticles were oxidised by a beam of O2 before landing on quartz and silicon wafers for use in catalytic, optical or electron spectroscopic experiments. This procedure resulted in fully oxidized nanoparticles (ex situ XPS). Thus in any given run the same material was deposited on both types of wafer, the deposition rate being continuously monitored by a quartz crystal microbalance.
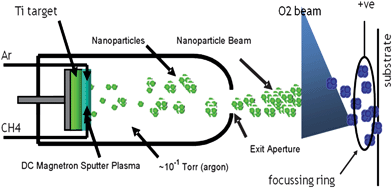 | ||
| Fig. 1 Nanoparticles synthesis and deposition. | ||
The as-prepared TiO2(C) thin films were optically transparent and amorphous. After calcination at 500 °C for 4 h in air, XRD showed complete conversion to anatase†.
The UV-Vis absorption spectra of a pure TiO2 film and two TiO2(C) films prepared by this method are shown in Fig. 2. Spectrum M1, pure TiO2, was identical with that obtained from a reference TiO2 thin film made by dip coating a quartz slide with Degussa P25 titania, thus validating the synthetic procedure. Film M2, with a low carbon content, differed little from pure TiO2 in regard to optical properties—the same was true of its photocatalytic properties (see below). However M3 showed enhanced optical absorption in the visible region including a pronounced ∼100 nm red shift of the band edge. These results demonstrate that doping with sufficient carbon by our procedure does induce visible light absorption, a necessary though not sufficient condition for visible light-induced photoactivity. This is an important point: often, dopant-induced visible light absorption by TiO2 does not result in photoactivity: the dopant must be present in the right chemical state as recent work on N-doping2,10 and B-doping11 clearly demonstrates.
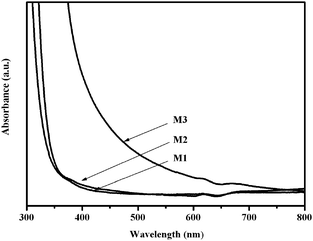 | ||
| Fig. 2 The UV-Vis absorption spectra of TiO2 (M1) and carbon-doped TiO2 (M2 and M3) thin films. | ||
The films' photocatalytic activities were evaluated using a recently described method for rapid assessment of such surfaces, invented and validated by Mills et. al.,4,13 based on the use of “intelligent ink”. The latter consisted of the appropriate mixture of the dye resazurin, hydroxyethyl cellulose and glycerol.4,13 The various samples were spin-coated with the ink (500 rpm for 8 s followed by 4000 rpm for 30 s)6 and their photocatalytic activity examined by illuminating them with visible light (≥420 nm; filtered 1 kW Xe lamp) whilst monitoring the attenuation of the dye absorption spectrum at 610–580 nm resulting from its photo-mineralization, most likely according to the mechanism proposed by Mills et al.4,13 As is apparent from Fig. 3, M1 (pure anatase) showed very low visible light-induced activity, consistent with negligible visible light absorption of this sample (Fig. 2). This suggests that surface sensitization by dye, although it cannot be completely excluded, is negligible in the present case. By comparison, M3 was far more active. The results were consistent for both reactant (resazurin) and reaction product (resofurin). Both of them are decomposed by superoxide radicals, which are generated on the surface of the anatase film.
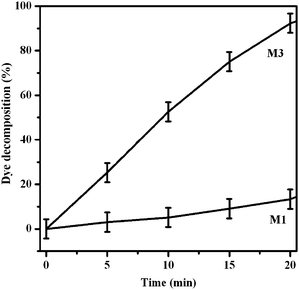 | ||
| Fig. 3 Photo-mineralization of resazurin under visible light irradiation for samples M1 and M3. Performance of inactive sample M2 (not shown) essentially the same as M1 (un-doped anatase film). | ||
Fig. 4 shows C 1s XP spectra obtained from samples M1, M2, and M3. All spectra exhibit a broad feature at ∼285–287 eV. Control experiments showed that this may be assigned to adventitious contamination of the support wafers by hydrocarbonaceous and oxygenated organic species14 present in the residual vacuum.
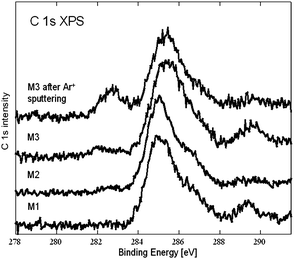 | ||
| Fig. 4 C 1s XP spectra of the samples. | ||
The emission at ∼289 eV corresponds to a carbonate.14 The weak feature at ∼283 eV in the M3 spectrum and barely visible in the M2 spectrum corresponds to carbon incorporated substitutionally in the TiO2 lattice.15–17 As shown by the top spectrum in Fig. 3, in situ surface cleaning of sample M3 by Ar+ sputtering removed the superficial carbonate contamination and strongly enhanced the ∼283 eV emission characteristic of substutional carbon. In view of the UV-Vis spectra and the photocatalytic results, we ascribe the observed visble light photoactivity to substitutionally incorporated carbon. Thus our method is capable of producing C-doped TiO2 films with carbon present in the right chemical state. Quantification of the XPS data via the C1s : Ti 2p intensity ratio and allowing for photoionization cross sections, yields an estimate of ∼0.5 atomic% for the amount of “good” carbon present in sample M3. Very recently we have produced boron-doped TiO2 thin films using B10H12 instead of CH4 as the source of dopant atoms. Interestingly, in this case too boron was incorporated in the correct chemical state11 for inducing visible light-activated photocatalysis. Maximum activity occurred at ∼1.0 atomic% B associated with an “active” boron component probably present substitutionally at oxygen sites. Inactive boron was present as boric oxide-like material.
Various mechanisms have been proposed to account for the visible light activity of anion-doped TiO2. In the case of nitrogen-doped anatase it has been shown that substitutionally doped N gives rise to localised N 2p acceptor states above the valence band8 which are the likely origin of visble light induced photocatalytic activity. Interestingly, and in good accord with the present results, recent theoretical results for C-doped TiO2 show that substitutionally doped carbon also gives rise to localised C 2p acceptor states, which induce visible light photoactivity.18
In summary, a versatile method has been developed that allows deposition of carbon-doped titania thin films on a wide range of solid substrates. The thickness, particle size and dopant concentration within the film may be varied in a controlled way, and extension to other dopant atoms is readily achieved. In the present case, carbon is incorporated in the desired chemical state resulting in visible light-activated photocatalytic activity.
Acknowledgements
We acknowledge financial support by the Korea Science and Engineering Foundation (Grant No. M06-2004-000-10026, S.I.) EC grant NMP3-CT-2006-032583.Notes and references
- A. Nakajima, K. Hashimoto, T. Watanabe, K. Takai, G. Yamauchi and A. Fujishima, Langmuir, 2000, 16(17), 7044 CrossRef CAS.
- H. M. Yates, M. G. Nolan, D. W. Sheel and M. E. Pemble, J. Photochem. Photobiol., A, 2006, 179, 213 CrossRef CAS.
- B. O'regan and M. Grätzel, Nature, 1991, 353(6346), 737 CrossRef CAS.
- A. Mills, J. S. Wang, M. Crow, G. Taglioni and L. Novella, J. Photochem. Photobiol., A, 2007, 187, 370 CrossRef CAS.
- A. Karuppasamy and A. Subrahmanyam, J. Appl. Phys., 2007, 101, 64318 CrossRef.
- Y. F. Zhu, L. Zhang, W. Q. Yao and L. L. Cao, Appl. Surf. Sci., 2000, 158, 32 CrossRef CAS.
- J. Medina-Valtierra, J. Garcia-Servin, C. Frausto-Reyes and S. Calixto, Appl. Surf. Sci., 2006, 252, 3600 CrossRef CAS.
- A. Mills, S. K. Lee and A. Lepre, J. Photochem. Photobiol., A, 2003, 155, 199 CrossRef CAS.
- C. K. Xu, R. Killmeyer, M. L. Gray and S. U. M. Khan, Electrochem. Commun., 2006, 8, 1650 CrossRef CAS.
- S. In, A. Orlov, F. Garcia, M. Tikhov, D. S. Wright and R. M. Lambert, Chem. Commun., 2006, 4236 RSC.
- S. In, A. Orlov, R. Berg, F. Garcia, S. Pedrosa-Jimenez, M. Tikhov, D. S. Wright and R. M. Lambert, J. Am. Chem. Soc., 2007, 129, 13790 CrossRef CAS.
- A. H. Kean and L. Allers, NSTI Nanotech Conference Proceedings, Boston, May 2006 Search PubMed.
- A. Mills, J. S. Wang, S. K. Lee and M. Simonsen, Chem. Commun., 2005, 2721 RSC.
- NIST X-ray Photoelectron Spectroscopy Database, Version 3.5 (National Institute of Standards and Technology, Gaithersburg, 2003); http://srdata.nist.gov/xps/ Search PubMed.
- H. Irie, S. Washizuka and K. Hashimoto, Thin Solid Films, 2006, 510, 21 CrossRef CAS.
- H. Irie, Y. Watanabe and K. Hashimoto, Chem. Lett., 2003, 32, 772 CrossRef CAS.
- S. W. Hsu, T. S. Yang, T. K. Chen and M. S. Wong, Thin Solid Films, 2007, 515, 3521 CrossRef CAS.
- T. Xu, C. Song, Y. Liu and G. Han, J. Zhejiang Univ. Sci. B, 2006, 7(4), 299 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Atomic force microscopy, PXRD, XPS and uv-vis spectroscopy. See DOI: 10.1039/b915060a |
| This journal is © The Royal Society of Chemistry 2009 |
