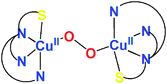
Thioether S-ligation in a side-on μ-η2:η2-peroxodicopper(II) complex†
Ga Young Parka, Yunho Leea, Dong-Heon Leeb, Julia S. Woertinkc, Amy A. Narducci Sarjeanta, Edward I. Solomonc and Kenneth D. Karlin*ad
aDepartment of Chemistry, The Johns Hopkins University, 3400 N. Charles Street, Baltimore, MD 21218, USA. E-mail: karlin@jhu.edu; Fax: +1 410-516-8420; Tel: +1 410-516-8027
bDepartment of Chemistry, Chonbuk National University, Jeonju 560-756, Korea
cDepartment of Chemistry, Stanford University, 333 Campus Drive, Stanford, CA 94305, USA
dDepartment of Bioinspired Science, Ewha Womans University, Seoul 120-750, Korea
First published on 4th November 2009
Abstract
[(ANS)CuI(CH3CN)]+ reacts with O2 giving [{(ANS)CuII}2(μ-η2:η2-O22−)]2+, νO–O = 731 cm−1, shown to possess S-thioether ligation, based on comparisons with analogues having all N-ligands or a –S(Ph) group. The finding is a rare occurrence and new for side-on O22− binding.
Copper(I)–dioxygen adducts or derived species pertinent to copper protein O2-binding and activation are currently of considerable interest in bioinorganic chemistry.1–4 The focus of this communication relates to the discovery and influence of thioether sulfur ligation in copper(I)–dioxygen adducts, a topic which has attracted more recent attention. This is due to the existence of important copper monooxygenases, namely dopamine β-monooxygenase (DβM) and peptidyl glycine α-hydroxylating monooxygenase (PHM), which serve important roles in neurotransmitter or regulatory hormone biosynthesis.5 The active-site in these proteins, where substrate binding and copper–dioxygen activation occur, is the so-called CuM site, which is ligated by two His and one Met ligands.5–7 Biochemical and chemical evidence suggests that O2 binds here and that a CuII–superoxo (O2˙−) dioxygen adduct or a derived CuII–hydroperoxo or cupryl (CuII–O˙) entity initiates substrate oxidation chemistry.5,8–12 In fact, the observance of an X-ray structure on a protein CuII–O2˙− complex,7 biochemical evidence5,8–10 and theoretical calculations9,10 show that the CuII–superoxo species is the one effecting a substrate hydrogen-atom abstraction reaction.
The vast majority of the literature deals with copper(I)–dioxygen chemistry and adducts and reactivity with systems containing all nitrogen ligands, typically bi-, tri-, or tetradentate entities.1–4,13,14 However, it is clearly important to develop an understanding of how thioether ligation influences the structure(s), spectroscopy, and reactivity of CuIn–(O2)-derived species.
Some work in this general area has occurred. A dicopper(I) complex with a Met-based ligand was synthesized by Casella and co-workers;15,16 reaction with O2 led to hydroxylation of a xylyl spacer group. Tolman and co-workers reported CuI–O2 chemistry with a N2S β-diketiminate ligand, but the S-donor does not coordinate to copper in the derived O2-adduct.17 Quite recently, Nicholas and co-workers synthesized a bis-imidazole-thioether tridentate N2S ligand while CuI-complex oxidation occurs, they did not observe CuI–O2 adducts.18–20
Recently, we reported the first copper–dioxygen adduct with thioether ligation, an “end-on”μ-1,2-peroxodicopper(II) complex, each copper ion possessing a tetradentate N3S ligand.21,22
As DβM and PHM employ N2S rather than N3S protein ligand donors, we were interested to look further, at new tridentate ligands. In this report, we describe copper(I)–O2 reactivity with ANS (Scheme 1), a linear N2S tridentate ligand. We also had previously studied copper(I)–O2 chemistry with the N3 ligand MeAN (Scheme 1), which readily forms a side-on binuclear peroxo complex [{(MeAN)CuII}2(μ-η2:η2-O22−)]2+ (2P), λmax = 360 nm (ε = 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1) and 540 nm (ε = 2500 M−1 cm−1).23 Our hypothesis was that an analogue, ANS, in which one alkylamine arm of MeAN is replaced by a thioether donor, might exhibit related chemistry. This turns out to be true, as we described here.
000 M−1 cm−1) and 540 nm (ε = 2500 M−1 cm−1).23 Our hypothesis was that an analogue, ANS, in which one alkylamine arm of MeAN is replaced by a thioether donor, might exhibit related chemistry. This turns out to be true, as we described here.
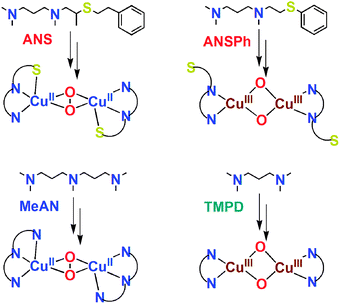 | ||
| Scheme 1 Ligands and their Cu2–O2 adducts. | ||
The copper(I) complex [(ANS)CuI(CH3CN)]+ (1A) (as B(C6F5)4− salt) was generated from [CuI(CH3CN)4]B(C6F5)4 addition to ANS, and characterized as a greenish white air-sensitive solid. [(ANS)CuII(Cl)2] (1B) was also synthesized. Both of these CuI and CuII complexes were characterized by X-ray diffraction (Fig. 1).‡ Notably, the S(thioether) coordination to copper ion occurs in both CuI and CuII complexes.
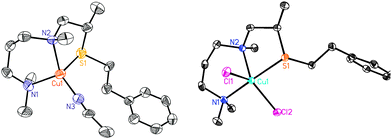 | ||
| Fig. 1 ORTEP diagram view (50% probability ellipsoids) of 1A and 1B. Left: X-ray structure of 1A: Cu1–N1, 2.056 Å; Cu1–N2, 2.122 Å; Cu1–N3, 1.935 Å; Cu1–S1, 2.292 Å. Right: X-ray structure of 1B: Cu1–N1, 2.071 Å; Cu1–N2, 2.136 Å; Cu1–S1, 2.387 Å, τ = 0.61.24 | ||
In order to describe the electronic environment of the ligand copper coordination complexes, while also comparing the environment of sulfur containing ANS (N2S) with MeAN (N3), CO adducts of [(ANS)CuI(CH3CN)]+ (1A) and [(MeAN)CuI]+ (2A) were generated and the electrochemistry via cyclic voltammetry of 1A and 2A were evaluated (Table 1). The IR spectrum of [(ANS)CuI-CO]+ (1-CO) shows that νCO = 2092 cm−1, significantly higher than that observed for [(MeAN)CuI-CO]+ (2-CO, 2079 cm−1). It is notable that νCO for the CuM sites in PHM and DβM (Table 1)25,26 are essentially the same as that of [(ANS)CuI-CO]+ (1-CO), indicating that 1A possesses a chemical environment reasonably mimicking that of protein active sites.
As for the ligand comparison, the results clearly indicate that MeAN is a better donor to copper(I) which then back-donates to its CO ligand to a greater extent, lowering νCO. The electrochemical behavior for 1Avs. 2A is consistent with findings for CO ligation and νCO values, as the strong donor ligand MeAN better stabilizes the higher oxidation state, giving a more negative E1/2 value (Table 1).
Oxygenation of 1A at −94 °C in acetone gives a dark orange species, λmax = 375 nm (ε = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 M−1) and 495 nm (ε = 1000 cm−1 M−1) (Fig. 2), features consistent with those of an μ-η2:η2-peroxodicopper(II) species.1 Thus, the complex is formulated as [{(ANS)CuII}2(μ-η2:η2-O22−)]2+ (1P). Confirmation derives from resonance Raman spectroscopic data with λex = 379.5 nm employing either 16O2 or 18O2 for complex generation: νCu–Cu = 273 cm−1 (Fig. 3A) and νO–O = 731 cm−1 (Δν(18O2) = −40 cm−1) (Fig. 3B). These vibrations are characteristic of a μ-η2:η2-peroxodicopper(II) species.1,27
000 cm−1 M−1) and 495 nm (ε = 1000 cm−1 M−1) (Fig. 2), features consistent with those of an μ-η2:η2-peroxodicopper(II) species.1 Thus, the complex is formulated as [{(ANS)CuII}2(μ-η2:η2-O22−)]2+ (1P). Confirmation derives from resonance Raman spectroscopic data with λex = 379.5 nm employing either 16O2 or 18O2 for complex generation: νCu–Cu = 273 cm−1 (Fig. 3A) and νO–O = 731 cm−1 (Δν(18O2) = −40 cm−1) (Fig. 3B). These vibrations are characteristic of a μ-η2:η2-peroxodicopper(II) species.1,27
![UV-Vis changes upon reaction of O2 with [(ANS)Cu(CH3CN)]+ (1A) (yellow) in acetone at −94 °C giving [{(ANS)CuII}2(μ-η2:η2-O22−)]2+ (1P) (red); warming to RT leads to a decomposition product (green).](/image/article/2010/CC/b918616f/b918616f-f2.gif) | ||
| Fig. 2 UV-Vis changes upon reaction of O2 with [(ANS)Cu(CH3CN)]+ (1A) (yellow) in acetone at −94 °C giving [{(ANS)CuII}2(μ-η2:η2-O22−)]2+ (1P) (red); warming to RT leads to a decomposition product (green). | ||
![rR spectra of [{(ANS)CuII}2(μ-η2:η2-O22−)]2+ (1P) in acetone with 16O2 (red) and 18O2 isotopic substitution (blue) in the region of νCu–Cu (A) and νO–O (B) (λex = 379.5 nm, 77 K, 5 mW power).](/image/article/2010/CC/b918616f/b918616f-f3.gif) | ||
| Fig. 3 rR spectra of [{(ANS)CuII}2(μ-η2:η2-O22−)]2+ (1P) in acetone with 16O2 (red) and 18O2 isotopic substitution (blue) in the region of νCu–Cu (A) and νO–O (B) (λex = 379.5 nm, 77 K, 5 mW power). | ||
These values are also similar to those observed for [{(MeAN)CuII}2(μ-η2:η2-O22−)]2+ (2P) (νO–O = 721 cm−1 and νCu–Cu = 268 cm−1),23 however the higher νO–O vibrational frequency of [{(ANS)CuII}2(μ-η2:η2-O22−)]2+ (1P) (731 cm−1), compared to that for MeAN complex 2P (721 cm−1), indicates that the O–O bond in 1P is stronger. Compared to MeAN, ANS is a weaker donor to copper resulting in increased peroxo π*σ donation to Cu and less back-bonding into the peroxo σ* resulting in a stronger peroxo O–O bond.28
The supposition that S(thioether) ligation occurs in [{(ANS)CuII}2(μ-η2:η2-O22−)]2+ (1P) derives from observations discussed above and the following argument. If the thioether donor did not bind to copper in 1P and was dangling, (as seen in Tolman’s N2S system),17 the remaining “bidentate amine” ligand (N2 portion of ANS) would result in formation of a bis-μ-oxo dicopper(III) species. As is already known, the copper(I) complex with N,N,N′,N′-tetramethyl-(1,3)-propanediamine (TMPD), [(TMPD)CuI(CH3CN)]+ (4A), which was described by Stack and co-workers,29 does exactly this (Scheme 1); νCu–O = 609 cm−1: Δν(18O2) = −28 cm−1. We also note that for a copper(I) complex with TMPD, E1/2 is very negative (−393 mV, vs. Fe(Cp)2+/0), consistent with it’s ability to form a bis-μ-oxo complex, stabilizing a high oxidation state copper(III) environment.
To provide further support for thioether ligation in 1P, we separately synthesized the ligand ANSPh (Scheme 1) and its Cu(I) complex [(ANSPh)CuI(CH3CN)]+ (3A). The large phenyl group of the ANSPh ligand, directly on the sulfur atom, inhibits sulfur coordination to copper ion in a Cu2–O2 adduct. This in fact is the case. The reaction of dioxygen with 3A at −94 °C in acetone gives a yellow bis-μ-oxo dicopper(III) species (3O), λmax = 409 nm (ε = 9000 cm−1 M−1) similar to typical bis-μ-oxo dicopper(III) species. Resonance Raman data for 3O (ESI†) show only a characteristic core vibration, νCu–O = 606 cm−1 (Δν (18O2) = −28 cm−1), suggesting a structure just like Stack’s TMPD complex (Scheme 1)29 and indicating the –SPh moiety does not coordinate. By inference, these results indicate that the S(thioether) donor in ANS must be able to coordinate to Cu(II) in [{(ANS)CuII}2(μ-η2:η2-O22−)]2+ (1P).
In summary, a side-on μ-η2:η2-peroxodicopper(II) species has been generated from the oxygenation of LCu(I) complex with N2S thioether ligand ANS. By comparison to the nature of the Cu2–O2 species generated with closely related ligands where either the S(thioether) is unable to coordinate or is absent (a bis-μ-oxo dicopper(III) complex), we can conclude that coordination of the S(thioether) donor within [{(ANS)CuII}2(μ-η2:η2-O22−)]2+ (1P) occurs. This is the first case of thioether coordination within a μ-η2:η2-peroxodicopper(II) structural type. This advance in copper–dioxygen chemistry further indicates that it is possible to study systems with S(thioether) ligation. Future investigations will focus on the effect of S(thioether) on reactivity of derived copper–dioxygen complexes,30 and on the design and study of systems where only one copper ion is present.
We are grateful to the NIH (K.D.K., GM28962; E.I.S., DK31450) for research support.
Notes and references
- L. M. Mirica, X. Ottenwaelder and T. D. P. Stack, Chem. Rev., 2004, 104, 1013 CrossRef CAS.
- E. A. Lewis and W. B. Tolman, Chem. Rev., 2004, 104, 1047 CrossRef CAS.
- S. Itoh, Curr. Opin. Chem. Biol., 2006, 10, 115 CrossRef CAS.
- L. Q. Hatcher and K. D. Karlin, J. Biol. Inorg. Chem., 2004, 9, 669 CAS.
- J. P. Klinman, J. Biol. Chem., 2006, 281, 3013 CAS.
- S. T. Prigge, R. E. Mains, B. A. Eipper and L. M. Amzel, Cell Mol. Life Sci., 2000, 57, 1236 CAS.
- S. T. Prigge, B. A. Eipper, R. E. Mains and L. M. Amzel, Science, 2004, 304, 864 CrossRef CAS.
- P. Chen, K. Fujisawa and E. I. Solomon, J. Am. Chem. Soc., 2000, 122, 10177 CrossRef CAS.
- P. Chen and E. I. Solomon, J. Am. Chem. Soc., 2004, 126, 4991 CrossRef CAS.
- P. Chen and E. I. Solomon, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 13105 CrossRef CAS.
- K. Yoshizawa, N. Kihara, T. Kamachi and Y. Shiota, Inorg. Chem., 2006, 45, 3034 CrossRef CAS.
- A. Crespo, M. A. Marti, A. E. Roitberg, L. M. Amzel and D. A. Estrin, J. Am. Chem. Soc., 2006, 128, 12817 CrossRef CAS.
- L. Q. Hatcher and K. D. Karlin, Adv. Inorg. Chem., 2006, 58, 131.
- T. Fujii, S. Yamaguchi, Y. Funahashi, T. Ozawa, T. Tosha, T. Kitagawa and H. Masuda, Chem. Commun., 2006, 4428 RSC.
- L. Casella, M. Gullotti, M. Bartosek, G. Pallanza and E. Laurenti, J. Chem. Soc., Chem. Commun., 1991, 1235 RSC.
- G. Alzuet, L. Casella, M. L. Villa, O. Carugo and M. Gullotti, J. Chem. Soc., Dalton Trans., 1997, 4789 RSC.
- N. W. Aboelella, B. F. Gherman, L. M. R. Hill, J. T. York, N. Holm, V. G. Young, C. J. Cramer and W. B. Tolman, J. Am. Chem. Soc., 2006, 128, 3445 CrossRef CAS.
- L. Zhou, D. Powell and K. M. Nicholas, Inorg. Chem., 2006, 45, 3840 CrossRef CAS.
- L. Zhou, D. Powell and K. M. Nicholas, Inorg. Chem., 2007, 46, 7789 CrossRef CAS.
- Some other studies involving nitrogen–thioether Cu complexes have been described: Reglier and co-workers demonstrated thioether ligand sulfoxidation from the reaction of a CuII–N3S complex with H2O2: L. Casella, M. Gullotti, M. Bartosek, G. Pallanza and E. Laurenti, J. Chem. Soc., Chem. Commun., 1991, 1235 Search PubMed . Kodera and co-workers characterized a CuII–OOH species from H2O2 reactivity with a thioether containing CuII complex: M. Kodera, T. Kita, I. Miura, N. Nakayama, T. Kawata, K. Kano and S. Hirota, J. Am. Chem. Soc., 2001, 123, 7715 RSC . Lee et al., reported ligand sulfoxidation with CuI–N2S(thioether) complex: Y. Lee, D. H. Lee, A. A. N. Sarjeant, L. N. Zakharov, A. L. Rheingold and K. D. Karlin, Inorg. Chem., 2006, 45, 10098 Search PubMed.
- L. Q. Hatcher, D. H. Lee, M. A. Vance, A. E. Milligan, R. Sarangi, K. O. Hodgson, B. Hedman, E. I. Solomon and K. D. Karlin, Inorg. Chem., 2006, 45, 10055 CrossRef CAS.
- D. H. Lee, L. Y. Q. Hatcher, M. A. Vance, R. Sarangi, A. E. Milligan, A. A. N. Sarjeant, C. D. Incarvito, A. L. Rheingold, K. O. Hodgson, B. Hedman, E. I. Solomon and K. D. Karlin, Inorg. Chem., 2007, 46, 6056 CrossRef CAS.
- H. C. Liang, C. X. Zhang, M. J. Henson, R. D. Sommer, K. R. Hatwell, S. Kaderli, A. D. Zuberbuhler, A. L. Rheingold, E. I. Solomon and K. D. Karlin, J. Am. Chem. Soc., 2002, 124, 4170 CrossRef CAS.
- A. W. Addison, T. N. Rao, J. Reedijk, J. Vanrijn and G. C. Verschoor, J. Chem. Soc., Dalton Trans., 1984, 1349 RSC.
- S. Jaron and N. J. Blackburn, Biochemistry, 1999, 38, 15086 CrossRef CAS.
- N. J. Blackburn, T. M. Pettingill, K. S. Seagraves and R. T. Shigeta, J. Biol. Chem., 1990, 265, 15383 CAS.
- rR spectra of 1P obtained with λex = 406.7 nm revealed the presence of a small fraction (∼15%) of a bis-μ-oxo dicopper(III) (νCu–O = 603 cm−1 (Δν(18O2) = −29 cm−1), explaining the small shoulder (near 410 nm) observed in the absorption spectrum (Fig. 2).
- M. J. Henson, M. A. Vance, C. X. Zhang, H. C. Liang, K. D. Karlin and E. I. Solomon, J. Am. Chem. Soc., 2003, 125, 5186 CrossRef CAS.
- V. Mahadevan, J. L. DuBois, B. Hedman, K. O. Hodgson and T. D. P. Stack, J. Am. Chem. Soc., 1999, 121, 5583 CrossRef CAS.
- Following thermal decomposition of [{(ANS)CuII}2(O22−)]2+ (1P) and [{(ANSPh)CuIII}2(O2−)2]2+ (3O) and work-up including demetallation, oxygenated ligand products, i.e., sulfoxides of ANS and ANSPh, were isolated in moderate yield. Further details and expanded studies will be the subject of a future report.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details of the structure determinations. CCDC 738906 and 738907. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b918616f |
‡ Suitable single crystals of [(ANS)CuI(CH3CN)]B(C6F5)4 (1A) and [(ANS)CuII(Cl)2] (1B) were mounted in Paratone-N oil on the end of a glass fiber and transferred to the N2 cold stream of an Oxford Diffraction Xcalibur3 system equipped with Enhance optics and a CCD detector. The frames were integrated and a face indexed absorption correction and an interframe scaling correction were also applied with the Oxford Diffraction CrysAlisRED software package (CrysAlis CCD, Oxford Diffraction Ltd., Version 1.171.27p5 beta). The structures were solved using direct methods and refined using the Bruker SHELXTL (v6.1) software package (Sheldrick, G.M. 2000). [(ANS)CuI(CH3CN)]B(C6F5)4 (1A): crystal data. C19H33CuN3S·C24BF20, M = 1078.13, orthorhombic, Pbca, T = 110 K, a = 20.3371(4) Å, b = 16.4920(3) Å, c = 6.8598(6) Å, V/Å3 = 9008.8(3), Z = 8, μ(Mo-Kα) = 0.65 mm−1, crystal size/mm = 0.78 × 0.25 × 0.12, 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 908 reflections measured, 10 908 reflections measured, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 274 unique (Rint = 0.083). The final R1 (I > 2σ(I)) was 0.091. CCDC 738906. [(ANS)CuII(Cl)2] (1B): crystal data. C17H30Cl2CuN2S, M = 428.93, monoclinic, P21/n, T = 110 K, a = 14.4850(6) Å, b = 9.3727(3) Å, c = 16.0378(6) Å, β = 115.278(5)°, V/Å3 = 1968.86 (15), Z = 4, μ(Mo-Kα) = 1.47 mm−1, crystal size/mm = 0.77 × 0.18 × 0.08, 12 274 unique (Rint = 0.083). The final R1 (I > 2σ(I)) was 0.091. CCDC 738906. [(ANS)CuII(Cl)2] (1B): crystal data. C17H30Cl2CuN2S, M = 428.93, monoclinic, P21/n, T = 110 K, a = 14.4850(6) Å, b = 9.3727(3) Å, c = 16.0378(6) Å, β = 115.278(5)°, V/Å3 = 1968.86 (15), Z = 4, μ(Mo-Kα) = 1.47 mm−1, crystal size/mm = 0.77 × 0.18 × 0.08, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 886 reflections measured, 4653 unique (Rint = 0.031). The final R1 (I > 2σ(I)) was 0.032. CCDC 738907. 886 reflections measured, 4653 unique (Rint = 0.031). The final R1 (I > 2σ(I)) was 0.032. CCDC 738907. |
| This journal is © The Royal Society of Chemistry 2010 |