Single and double substrate insertion into the Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/h2_char_e001.gif) Nα bonds of terminal titanium hydrazides†
Nα bonds of terminal titanium hydrazides†
Pei-Jen Tionga, A. Daniel Schofielda, Jonathan D. Selbya, Ainara Novab, Eric Clot*b and Philip Mountford*a
aChemistry Research Laboratory, University of Oxford, Mansfield Road, Oxford, UK OX1 3TA. E-mail: philip.mountford@chem.ox.ac.uk
bInstitut Charles Gerhardt Montpellier, CNRS 5253, Université Montpellier 2, cc 1501, Place Eugène Bataillon, F-34095 Montpellier Cedex 5, France. E-mail: eric.clot@univ-montp2.fr
First published on 19th November 2009
Abstract
Nitriles, CO2 and isocyanates undergo net single or double insertion reactions into the Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Nα multiple bonds of terminal titanium hydrazides. These are the first such examples of this type of reactivity for any transition metal hydrazide complex.
Nα multiple bonds of terminal titanium hydrazides. These are the first such examples of this type of reactivity for any transition metal hydrazide complex.
Studies of the synthesis and reactivity of transition metal hydrazides, (L)M
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNR2, have been of sustained interest and activity for over 25 years. Historically, this is because of their relevance to the biological conversion (fixation) of N2 to ammonia.1–4 For this reason, much of the early work was based around Group 6 systems4–6 for which M
NNR2, have been of sustained interest and activity for over 25 years. Historically, this is because of their relevance to the biological conversion (fixation) of N2 to ammonia.1–4 For this reason, much of the early work was based around Group 6 systems4–6 for which M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Nα–NβR2 group reactivity is characterised by reactions of the Nβ atom (quaternarisation or (for R = H) N–H bond chemistry) and/or Nα–Nβ reductive cleavage. Although initial reactivity reports of Group 4 hydrazides appeared some time ago,7,8 there has recently been a surge in activity and output in this area,9–21 and a number of new reactions of the M
Nα–NβR2 group reactivity is characterised by reactions of the Nβ atom (quaternarisation or (for R = H) N–H bond chemistry) and/or Nα–Nβ reductive cleavage. Although initial reactivity reports of Group 4 hydrazides appeared some time ago,7,8 there has recently been a surge in activity and output in this area,9–21 and a number of new reactions of the M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNR2 functional group have been discovered. These include the cycloaddition of alkynes (as in I, Fig. 1) and heteroalkynes to the M
NNR2 functional group have been discovered. These include the cycloaddition of alkynes (as in I, Fig. 1) and heteroalkynes to the M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Nα multiple bond,21 and Nα–Nβ bond insertion reactions with several substrates, including alkynes18,21 and isocyanides (e.g. forming II and III, Fig. 1).16,22 The reactions of Ti(N2NMe)(NNPh2)(py) (N2NMe = MeN(CH2CH2NSiMe3)2) which lead to “insertion” products II and III can be viewed as Nα atom migration processes.
Nα multiple bond,21 and Nα–Nβ bond insertion reactions with several substrates, including alkynes18,21 and isocyanides (e.g. forming II and III, Fig. 1).16,22 The reactions of Ti(N2NMe)(NNPh2)(py) (N2NMe = MeN(CH2CH2NSiMe3)2) which lead to “insertion” products II and III can be viewed as Nα atom migration processes.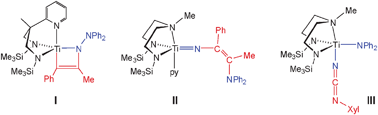 | ||
Fig. 1 Reaction products of Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNPh2 bonds with alkynes (I, II) and XylNC (III): M NNPh2 bonds with alkynes (I, II) and XylNC (III): M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Nα cycloaddition and Nα–Nβ bond cleavage. Nα cycloaddition and Nα–Nβ bond cleavage. | ||
Just as Group 6 hydrazides are important because of their structural and functional relevance to biological N2 fixation, Group 4 hydrazides are closely connected with the important area of catalytic N–C bond formation.11,23–25 Metallacycles analogous to I are intermediates in the metal-catalysed conversion of alkynes and hydrazines to hydrazones (i.e., hydrohydrazination: double N–H addition across a C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C multiple bond11,23–25). The Nα–Nβ bond insertion compound II and its homologues are intermediates in the diamination of internal alkynes using Ph2NNH2 (N–N addition across a C
C multiple bond11,23–25). The Nα–Nβ bond insertion compound II and its homologues are intermediates in the diamination of internal alkynes using Ph2NNH2 (N–N addition across a C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C multiple bond).18,21
C multiple bond).18,21
The fundamental paradigm of both hydrohydrazination and diamination is net transfer of an “NNR2” moiety from a metal hydrazide to an unsaturated substrate. In every case, the final Ti–Nα bond breaking step only occurs via protonolysis using an incoming R2NNH2. As part of our continuing interest in metal hydrazide and related chemistry, we have started to develop new approaches to “NNR2” functional group transfer reactions of these emerging new classes of early transition metal compound. We report here the first M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Nα bond insertion reactions of any metal hydrazide compound.
Nα bond insertion reactions of any metal hydrazide compound.
Reaction of the recently reported18 terminal hydrazides Cp*Ti{MeC(NiPr)2}(NNR2) (R = Ph (1a) or Me (1b)) with an excess of CO2 gave the dicarboxylate complexes Cp*Ti{MeC(NiPr)2}{OC(O)N(NR2)C(O)O} (R = Ph (2a) or Me (2b)) in good yields. The spectroscopic and other data for 2a and 2b are consistent with the Cs symmetric “double insertion” products depicted in Scheme 1, and the X-ray structure of 2a (Fig. 2) confirms this in the solid state.†
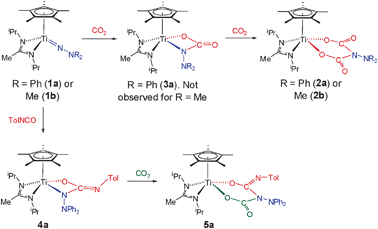 | ||
| Scheme 1 Cycloaddition and cycloaddition–insertion reactions of Cp*Ti{MeC(NiPr)2}(NNR2) with heterocumulenes. All reactions are at room temperature in toluene or C6H6 with 1 atm CO2 (where relevant). | ||
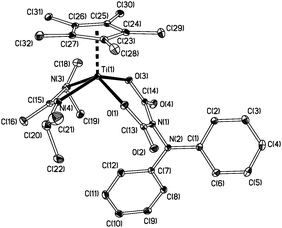 | ||
| Fig. 2 Displacement ellipsoid plot (20%) of Cp*Ti{MeC(NiPr)2}{OC(O)N(NPh2)C(O)O} (2a). Ti(1)–O(1) 1.937(2), Ti(1)–O(3) 1.951(2), Ti(1)–Cpcent 2.051, Ti(1)–N(3) 2.059(2), Ti(1)–N(4) 2.090(2), N(1)–N(2) 1.394(3), C(13)–O(2) 1.206(3), C(14)–O(4) 1.218(3) Å. | ||
As Fig. 2 shows, two molecules of CO2 have effectively inserted into the Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Nα (formally triple17) bond of 1a. This type of transformation is very rare in metal–nitrogen multiple bond chemistry in general,26,27 and without precedent in metal hydrazide chemistry at all. While the OC(O)N(NR2)C(O)O moiety in 2a and 2b is a new structural unit in transition metal chemistry, we note Chirik’s recent reports of metal-bound 1,1- or 1,2-NN(CO2)2 ligands prepared from metallocene–N2 complexes and CO2.28,29
Nα (formally triple17) bond of 1a. This type of transformation is very rare in metal–nitrogen multiple bond chemistry in general,26,27 and without precedent in metal hydrazide chemistry at all. While the OC(O)N(NR2)C(O)O moiety in 2a and 2b is a new structural unit in transition metal chemistry, we note Chirik’s recent reports of metal-bound 1,1- or 1,2-NN(CO2)2 ligands prepared from metallocene–N2 complexes and CO2.28,29
When the reaction between 2a and CO2 was followed by 1H NMR an intermediate cycloaddition product 3a (Scheme 1) was observed. Subsequent scale up led to isolated 3a in 54% yield. Reaction of 3a with CO2 quantitatively forms 2a. No intermediate analogous to 3a was observed for 1b at room temperature. The stepwise formation of 2a from 1avia3a suggested that mixed Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Nα insertion products should be accessible. Reaction of 1a with TolNCO gave 4a (Scheme 1), and subsequent addition of CO2 to 4a gave the mixed double insertion product 5a in 37% isolated yield (quantitative when followed by 1H NMR). The IR spectrum of 5a shows ν(C
Nα insertion products should be accessible. Reaction of 1a with TolNCO gave 4a (Scheme 1), and subsequent addition of CO2 to 4a gave the mixed double insertion product 5a in 37% isolated yield (quantitative when followed by 1H NMR). The IR spectrum of 5a shows ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and ν(C
O) and ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NTol) bands at 1699 and 1628 cm−1, respectively.
NTol) bands at 1699 and 1628 cm−1, respectively.
 | (1) |
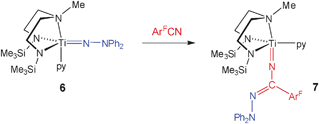
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Nα insertion reaction starting from the previously reported Ti(N2NMe)(NNPh2)(py) (6) mentioned in the introductory paragraphs. In this case a single molecule of ArFCN (ArF = C6F5) has inserted into the Ti
Nα insertion reaction starting from the previously reported Ti(N2NMe)(NNPh2)(py) (6) mentioned in the introductory paragraphs. In this case a single molecule of ArFCN (ArF = C6F5) has inserted into the Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Nα bond of 6 forming the hydrazonamide derivative 7. The solid state structure of 7 (Fig. 3) confirmed the formation of the new hydrazonamide fragment NC(ArF)NNPh2. The Ti(1)–N(1) distance of 1.781(1) Å is consistent with substantial multiple bond character, and is only slightly longer than Ti
Nα bond of 6 forming the hydrazonamide derivative 7. The solid state structure of 7 (Fig. 3) confirmed the formation of the new hydrazonamide fragment NC(ArF)NNPh2. The Ti(1)–N(1) distance of 1.781(1) Å is consistent with substantial multiple bond character, and is only slightly longer than Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Nα (1.733(5) Å) in 6 itself.
Nα (1.733(5) Å) in 6 itself.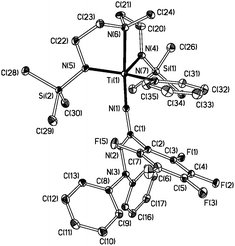 | ||
| Fig. 3 Displacement ellipsoid plot (20%) of Ti(N2NMe){NC(ArF)NNPh2}(py) (7). Ti(1)–N(1) 1.781(1), Ti(1)–N(4) 1.964(1), Ti(1)–N(5) 1.964(1), C(1)–N(1) 1.338(2), C(1)–N(2) 1.308(2), C(1)–C(2) 1.514(2), N(2)–N(3) 1.441(2) Å. | ||
Compound 7 is the first example of a terminal hydrazonamide complex. Mono- and di-metallated hydrazonamides have been reported by sequential reaction of AlMe3 or dialkyl zincs with Me2NNH2 and MeCN,30,31 but formed polynuclear clusters. Since the metal-bound hydrazonamide moiety in 7 still possesses a Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N multiple bond, further reaction chemistry of this moiety should be possible, allowing development of this hitherto unexplored functional group.
N multiple bond, further reaction chemistry of this moiety should be possible, allowing development of this hitherto unexplored functional group.
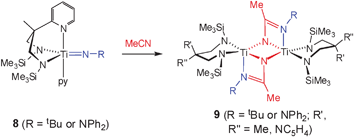
The reactions of metal hydrazido (or indeed imido) complexes with nitriles are almost without precedent, and only one previous system has been described (eqn (2)).21,32 This leads to the dimeric products 9 on reaction of Ti(N2Npy)(NR)(py) (8, R = tBu or NPh2) with MeCN. Terminal hydrazonamides analogous to 7 were not observed. Interestingly, when 6 was reacted with MeCN or PhCN equilibrium mixtures of unreacted starting materials and unidentified product(s) were observed.
To probe the factors and mechanism leading to the formation of the insertion product 7, we carried out DFT(B3PW91) calculations †on the model system Ti(N2SiH3NMe)(NNMe2)(py) (6Q, N2SiH3NMe = MeN(CH2CH2NSiH3)2) and ArFCN (Fig. 4). All energies given in the text are Gibbs free energies (T = 298 K, P = 1 atm) estimated from separated 6Q and nitrile.
 | (2) |
![Energy profile for reaction of Ti(N2SiH3NMe)(NNMe2)(py) (6Q) with ArFCN. All energies are ΔG in kcal mol−1. [Ti] = Ti(N2SiH3NMe).](/image/article/2010/CC/b920090h/b920090h-f4.gif) | ||
| Fig. 4 Energy profile for reaction of Ti(N2SiH3NMe)(NNMe2)(py) (6Q) with ArFCN. All energies are ΔG in kcal mol−1. [Ti] = Ti(N2SiH3NMe). | ||
The reactions of 6Q with MeCN and PhCN were also examined computationally. In contrast to the situation with the fluorinated substrate, the products of MeCN (7Q_Me) and PhCN (7Q_Ph) insertion into the Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Nα bond were computed to be endoergic (ΔG = 3.1 kcal mol−1 for MeCN; 5.1 kcal mol−1 for PhCN). The η2-RCN adducts (Int2_Me and Int2_Ph) and the cycloaddition products (Int3_Me and Int3_Ph) have also energies above those of the reactants (ΔG = 6.3 kcal mol−1 for Int2_Me; ΔG = 8.5 kcal mol−1 for Int2_Ph; ΔG = 0.2 kcal mol−1 for Int3_Me; ΔG = 3.2 kcal mol−1 for Int3_Ph). There is thus no thermodynamic driving force towards formation of the insertion products 7Q_Me or 7Q_Ph. This could explain the experimental observation of equilibrium mixtures of products and starting materials in the reactions of the real system 6 with MeCN and PhCN. Note that self-trapping (dimerisation) of species of the type Int3 would lead to dimeric compounds such as 9 (eqn (2)) observed experimentally. An NBO analysis of the electronic structure of 7Q indicated that the electron-withdrawing character of the ArF group plays a crucial role in formation of the insertion product through stabilisation of the Nα lone pair. Accordingly, the insertion product with CF3CN (7Q_CF3) was computed to lie below separated 6Q and CF3CN by ΔG = −11.6 kcal mol−1.
Nα bond were computed to be endoergic (ΔG = 3.1 kcal mol−1 for MeCN; 5.1 kcal mol−1 for PhCN). The η2-RCN adducts (Int2_Me and Int2_Ph) and the cycloaddition products (Int3_Me and Int3_Ph) have also energies above those of the reactants (ΔG = 6.3 kcal mol−1 for Int2_Me; ΔG = 8.5 kcal mol−1 for Int2_Ph; ΔG = 0.2 kcal mol−1 for Int3_Me; ΔG = 3.2 kcal mol−1 for Int3_Ph). There is thus no thermodynamic driving force towards formation of the insertion products 7Q_Me or 7Q_Ph. This could explain the experimental observation of equilibrium mixtures of products and starting materials in the reactions of the real system 6 with MeCN and PhCN. Note that self-trapping (dimerisation) of species of the type Int3 would lead to dimeric compounds such as 9 (eqn (2)) observed experimentally. An NBO analysis of the electronic structure of 7Q indicated that the electron-withdrawing character of the ArF group plays a crucial role in formation of the insertion product through stabilisation of the Nα lone pair. Accordingly, the insertion product with CF3CN (7Q_CF3) was computed to lie below separated 6Q and CF3CN by ΔG = −11.6 kcal mol−1.
The proposed mechanism is reminiscent of a classical Chauvin-type metathesis reaction,33 in that it proceeds through [2+2] cycloaddition (Int3) and reverse cycloaddition steps, leading to 7Q. However, whereas in alkyne or alkene metathesis (mediated through metal alkylidyne or alkylidene species), separation of the bond-exchanged partners can occur, this does not happen in the reactions of hydrazides and nitriles since the required species, Ti(N2NMe)(py)(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N˙) and Me2NN
N˙) and Me2NN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C˙ArF, are evidently radical-like in nature. Thus eqn (1) and Fig. 4 can be thought of as an “arrested” or “frustrated” Ti
C˙ArF, are evidently radical-like in nature. Thus eqn (1) and Fig. 4 can be thought of as an “arrested” or “frustrated” Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N/C
N/C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N metathesis reaction.
N metathesis reaction.
In conclusion, we have reported the first M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Nα insertion reactions of any transition metal hydrazide. Two different mechanisms have been observed: (i) cycloaddition–insertion leading to homo- or cross-coupled bis(heterocumulene) derivatives; (ii) “arrested” metathesis giving single-substrate insertion, again with Ti
Nα insertion reactions of any transition metal hydrazide. Two different mechanisms have been observed: (i) cycloaddition–insertion leading to homo- or cross-coupled bis(heterocumulene) derivatives; (ii) “arrested” metathesis giving single-substrate insertion, again with Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Nα bond cleavage. In this latter case (7, eqn (1)), a new Ti
Nα bond cleavage. In this latter case (7, eqn (1)), a new Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N multiple bond is formed which could in principle be a site of additional functionalisation. These results will be of benefit in developing Group 4 based substrate functionalisation chemistry via hydrazide intermediates.
N multiple bond is formed which could in principle be a site of additional functionalisation. These results will be of benefit in developing Group 4 based substrate functionalisation chemistry via hydrazide intermediates.
We thank the EPSRC for scholarships to A.D.S. and J.D.S., the Malaysian Higher Education Ministry for a scholarship to P.-J.T., and the Spanish MICINN for a MEC postdoctoral fellowship to A.N.
Notes and references
- R. R. Schrock, Angew. Chem., Int. Ed., 2008, 47, 5512 CrossRef CAS.
- R. R. Schrock, Acc. Chem. Res., 2005, 38, 955 CrossRef.
- B. A. MacKay and M. D. Fryzuk, Chem. Rev., 2004, 104, 385 CrossRef.
- M. Hidai, Coord. Chem. Rev., 1999, 185–186, 99 CrossRef CAS.
- M. D. Fryzuk, Chem. Rec., 2003, 3, 2 CrossRef CAS.
- M. P. Shaver and M. D. Fryzuk, Adv. Synth. Catal., 2003, 345, 1061 CrossRef CAS.
- P. J. Walsh, M. J. Carney and R. G. Bergman, J. Am. Chem. Soc., 1991, 113, 6343 CrossRef CAS.
- A. J. Blake, J. M. McInnes, P. Mountford, G. I. Nikonov, D. Swallow and D. J. Watkin, J. Chem. Soc., Dalton Trans., 1999, 379 RSC.
- D. J. Mindiola, Angew. Chem., Int. Ed., 2008, 47, 1557 CrossRef CAS.
- Y. Li, Y. Shi and A. L. Odom, J. Am. Chem. Soc., 2004, 126, 1794 CrossRef CAS.
- S. Banerjee and A. L. Odom, Organometallics, 2006, 25, 3099 CrossRef CAS.
- S. Patel, Y. Li and A. L. Odom, Inorg. Chem., 2007, 46, 6373 CrossRef CAS.
- K. Weitershaus, H. Wadepohl and L. H. Gade, Organometallics, 2009, 28, 3381 CrossRef CAS.
- T. Gehrmann, J. Lloret Fillol, H. Wadepohl and L. H. Gade, Angew. Chem., Int. Ed., 2009, 48, 2152 CrossRef CAS.
- H. Herrmann, J. L. Fillol, T. Gehrmann, M. Enders, H. Wadepohl and L. H. Gade, Chem.–Eur. J., 2008, 14, 8131 CrossRef CAS.
- H. Herrmann, J. L. Fillol, H. Wadepohl and L. H. Gade, Angew. Chem., Int. Ed., 2007, 46, 8426 CrossRef CAS.
- T. B. Parsons, N. Hazari, A. R. Cowley, J. C. Green and P. Mountford, Inorg. Chem., 2005, 44, 8442 CrossRef CAS.
- J. D. Selby, C. D. Manley, M. Feliz, A. D. Schwarz, E. Clot and P. Mountford, Chem. Commun., 2007, 4937 RSC.
- A. J. Clulow, J. D. Selby, M. G. Cushion, A. D. Schwartz and P. Mountford, Inorg. Chem., 2008, 47, 12049 CrossRef CAS.
- J. D. Selby, C. D. Manley, A. D. Schwarz, E. Clot and P. Mountford, Organometallics, 2008, 27, 6479 CrossRef CAS.
- J. D. Selby, C. Schulten, A. D. Schwarz, A. Stasch, E. Clot, C. Jones and P. Mountford, Chem. Commun., 2008, 5101 RSC.
- A. D. Schofield and P. Mountford, unpublished results.
- C. Cao, Y. Shi and A. L. Odom, Org. Lett., 2002, 4, 2853 CrossRef CAS.
- J. T. Ciszewski, J. F. Harrison and A. L. Odom, Inorg. Chem., 2004, 43, 3605 CrossRef CAS.
- A. L. Odom, Dalton Trans., 2005, 225 RSC.
- D. S. Glueck, J. Wu, F. J. Hollander and R. G. Bergman, J. Am. Chem. Soc., 1991, 113, 2041 CrossRef CAS.
- A. E. Guiducci, C. L. Boyd, E. Clot and P. Mountford, Dalton Trans., 2009, 5960 RSC.
- W. H. Bernskoetter, E. Lobkovsky and P. J. Chirik, Angew. Chem., Int. Ed., 2007, 46, 2858 CrossRef.
- D. J. Knobloch, H. E. Toomey and P. J. Chirik, J. Am. Chem. Soc., 2008, 130, 4248 CrossRef CAS.
- V. C. Gibson, C. Redshaw, A. J. P. White and D. J. Williams, Angew. Chem., Int. Ed., 1999, 38, 961 CrossRef CAS.
- M. R. Elsegood and C. Redshaw, Chem.–Eur. J., 2008, 14, 3530 CrossRef CAS.
- S. M. Pugh, D. J. M. Trösch, D. J. Wilson, A. Bashall, F. G. N. Cloke, L. H. Gade, P. B. Hitchcock, M. McPartlin, J. F. Nixon and P. Mountford, Inorg. Chem., 2000, 19, 3205 CAS.
- Y. Chauvin, Angew. Chem., Int. Ed., 2006, 45, 3740 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Characterisation and crystallographic data, and computational details. CCDC 752046 and 752047. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b920090h. |
| This journal is © The Royal Society of Chemistry 2010 |
