Synthesis and crystal structure of two new heterometallic thioantimonates(III) [Ni(pda)2]CuI4SbIII2S6 and [Ni(dien)2]CuISbIII3S6†,‡
Meng
Zhang
ab,
Tianlu
Sheng
a,
Xin
Wang
a,
Shengmin
Hu
a,
Ruibiao
Fu
a,
Jianshan
Chen
a,
Yimin
He
a,
Zhentao
Qin
a,
Chaojun
Shen
a and
Xintao
Wu
*a
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, China. E-mail: wxt@fjirsm.ac.cn.; Fax: +86-591-83714946; Tel: +86-591-83719238
bGraduate University of the Chinese Academy of Sciences, Beijing, 100039, China
First published on 28th August 2009
Abstract
Two different dimensional inorganic–organic hybrid heterometallic sulfide, [Ni(pda)2]Cu4Sb2S6 (1) and [Ni(dien)2]CuSb3S6 (2) were obtained by using 1,2-propanediamine (pda) and diethylenetriamine (dien) as the ligand, respectively.
Chalcogenidometalate chemistry is an attractive subject due to compounds' integrating properties of classical electronics, optics and semiconductor materials associated with unique topological structures,1 and the potential application as catalysts2 or as superconductors.3 The design and synthesis of chalcogenidometalates have received considerable attention4 during the last decades. To design and synthesize more compounds with various configurations, it is very important to understand which factors affect the structures of chalcogenidometalates. We have to some extent studied how the structures of the sulfur-containing transition metal polymers are influenced by the size or charge of cations.4 By using different cations such as [Nd(DMF)8]3+ and [La(DMF)8]3+, we have successfully synthesized and characterized a large number of complexes with different structures.5 Now we further extend our interest to the study of main-group sulfides, thioantimonates.6
During the last few years a large number of thioantimonates have been synthesized under solvothermal conditions with organic amines as structure-directing agents.6–27 In some of these compounds another transition metal ion (TMn+) is incorporated into the SbSx network forming a ternary TM–Sb–S anionic framework.7–17 The protonated amine normally acts as a cation in most of these templated TM-thioantimonates, whereas in a few compounds such as [M(NH3)6]Cu8Sb3S13 (M = Mn, Fe, Ni), [Fe(NH3)6]AgSbS4 and [Co(en)3]CoSb4S8 a transition metal complex serves as counterion directing to the anionic structures.18,19
Herein we present two amine-templated nickel-copper-thioantimonates(III), [Ni(pda)2]Cu4Sb2S6 (1) and [Ni(dien)2]CuSb3S6 (2). Compound 1, to the best of our knowledge, is the first inorganic–organic hybrid example of a 3D hetero-trimetallic sulfide, in which both NiII and CuI are incorporated into the SbSx framework. Compound 2 consists of large 18-membered Cu2Sb7S9 and small 6-membered CuSb2S3 hetero-rings.
Both compounds [Ni(pda)2]Cu4Sb2S6 (1) and [Ni(dien)2]CuSb3S6 (2) were synthesized in Teflon-lined steel autoclaves under solvothermal conditions. In the synthesis progress, a mixture of Sb (1 mmol), S (4 mmol), CuCl2·2H2O (1 mmol), NiCl2·6H2O (1 mmol) and pda (5 ml) for 1, and a mixture of Sb (1 mmol), S (3 mmol), Cu (1 mmol), NiCl2·6H2O (1 mmol) and water solution of dien (60%, 5 ml) for 2 were, respectively, heated to 140 °C for 6 d, followed by cooling to room temperature in 3 h. The reacted mixtures were filtered, washed with ethanol and water, successively dried in air, yielding 1 and 2. The majority of the red crystal 1 was contaminated, all attempts to purify the crystals and increase the yield unfortunately failed, and we could only physically pick out several clear crystals of 1.
Single crystal X-ray analysis revealed that compound 1 is an amine-templated 3D hetero-trimetallic sulfide, the structure is shown in Fig. 1–3. As shown in Fig. 1, the Ni2+ ion located on an inversion centre is coordinated by four N atoms of two pda ligands, the Ni2+ ion and four N atoms being in the same plane. The Ni–N bond distances are in the range of 2.077(11)–2.079(11) Å and comparable to those in the other thioantimonates(III).20,21 Furthermore, the Ni2+ ion is surrounded by the other two S atoms from the 2D (Cu4Sb2S62−)n sheets (Fig. 2), resulting in the 3D configuration of compound 1 (Fig. 3). The Ni2+ ion is in an ideal octahedral coordination and the Ni–S bond lengths are 2.633(4) Å, which are comparable to those in complex [Ni(tren)]Sb2S49. The crystallographically unique Sb(1) atom is coordinated by three S atoms to form a SbS3 trigonal pyramid. The Sb–S distances range from 2.435(4) to 2.438(4) Å and S–Sb–S angles from 99.48(13) to 99.68(13)°, which are common for SbS3 trigonal pyramids in thioantimonates.22,23 Both the Cu(1) and Cu(2) atoms are surrounded by three S atoms yielding two nearly trigonal planar CuS3 moieties (the Cu(1) and Cu(2) atoms are 0.1760 and 0.0740Å above the S3 planes, respectively). The Cu–S bond lengths scatter from 2.255(4) to 2.278(4) Å and the S–Cu–S angles from 113.00(15) to 127.36(16)°. These values are in agreement with those found in many templated copper-thioantimaonates.10,14 The S(2) and S(3) atoms present a trigonal pyramid coordination by one Sb and two Cu atoms, while S(1) atoms are tetrahedrally coordinated by one Sb, one Ni and two Cu atoms. The tetrahedral coordination of the S atom has been seldom observed in amine-templated copper-thioantimaonates. The 2D (Cu4Sb2S62−)n sheet of compound 1 consists of 6-membered Cu2SbS3 rings (Fig. 2) and the inter-sheet separations are about 4.6201Å, which is shorter than that of other amine-templated copper-thioantimonates.12,16
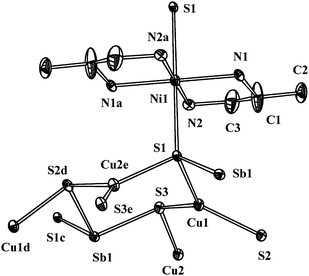 | ||
| Fig. 1 Local coordination of the framework atoms of 1 showing the atom-labeling scheme and thermal ellipsoids at 25% probability. Symmetry code: a: 1 − x, 1 − y, 1 − z; b: 1 + x, y, z; c: −1 + x, y, z; d: −1/2 + x, 3/2 – y, −1/2 + z; e: 1/2 + x, 3/2 – y, −1/2 + z. | ||
 | ||
| Fig. 2 Structure of the 2D (Cu4Sb2S62−)n sheet in compound 1. | ||
 | ||
| Fig. 3 3D structure of compound 1. | ||
Compound 2 consists of isolated transition-metal-amine [Ni(dien)2]2+ cations and 2D (CuSb3S62−)n anionic layers, the structure are shown in Fig. 4–6. In 2, as shown in Fig. 4, there are two independent Ni atoms each one lying on an inversion centre. The Ni2+ ion is coordinated by six N atoms of two dien ligands, forming a standard octahedron. The Ni–N bond lengths scatter from 2.087(3) to 2.155(4) Å with N–Ni–Ntran angles of 180°. The values are comparable to other thioantimonates.20,21 The (CuSb3S62−)n anionic layers contain crystallographically distinct one Cu, three Sb and six S atoms. All the Sb(1), Sb(2) and Sb(3) atoms are coordinated by three S atoms to form SbS3 trigonal pyramids. The Sb–S bond distances vary from 2.3522(11) to 2.4767(13) Å and the S–Sb–S angles from 86.57(4) to 101.61(5)°. The Cu(1) atom is surrounded by three S atoms to form a nearly trigonal planar CuS3 moiety, the Cu(1) atom being 0.0865 Å above the S3 plane. The Cu–S distances range from 2.2531(13) to 2.3137(13)Å and the S–Cu–S angles from 117.12(5) to 122.12(5)°. All the above distances (Sb–S, Cu–S) and angles (S–Sb–S, S–Cu–S) are similar to those found in other thioantimonates.12,16 Vertex-sharing of the SbS3 trigonal pyramids and the CuS3 moieties yield the 2D (CuSb3S62−)n anionic layers which lie parallel to the (100) plane. The interlayer distance is about 13.5 Å, which is longer than that of other amine-templated copper-thioantimonates.12,16 In the (CuSb3S62−)n anionic network, as shown in Fig. 5, all S atoms are doubly coordinated. The 2D structure is composed of 6-membered CuSb2S3 rings (different from the Cu2SbS3 rings in compound 1) and 18-membered Cu2Sb7S9 rings. The large 18-membered Cu2Sb7S9 ring is about 8.1290(13) × 6.1292(10) Å (from coordinate to coordinate), this is the largest pore in the amine-templated metal-thioantimonates so far.18 The anionic layers stack onto each other along the [100] direction, and the [Ni(dien)2]2+ cations lie between the anionic layers (Fig. 6).
 | ||
| Fig. 4 Local coordination of the framework atoms and two independent cations of 2 showing the atom-labeling scheme and thermal ellipsoids at 25% probability. Symmetry code: a: x, −1 + y, z; b: x, 1/2 − y, −1/2 + z; c: x, 1 + y, z; d: −x, 1 – y, 1 – z. | ||
 | ||
| Fig. 5 Structure of the (CuSb3S62−)n anionic layer with 18-membered Cu2Sb7S9 hetero-ring in compound 2. | ||
 | ||
| Fig. 6 Structure of compound 2. | ||
For most copper-thioantimonates, protonated amines are used as structure-directing agents to modify the structure of the anionic framework,10–12,16 and the anionic structure usually show a layered structure. In our work, metal complex cations are utilized to stabilize a chalcogen-containing anionic framework. For compound 1, pda serves as the chelating amine. Possibly due to steric hindrance, the Ni2+ ion is only planar coordinated by four N atoms of two pda ligands, and the remaining two coordination sites are further occupied by two S atoms from the 2D (Cu4Sb2S62−)n sheets composed of only 6-membered Cu2SbS3 rings. Hence, both NiII and CuI are incorporated into the SbSx framework to form the amine-templated 3D hetero-trimetallic sulfide, and the Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu
Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sb
Sb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S ratio is 1
S ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6. It should be noted that in amine-templated thioantimonates the Cu atoms tend to be coordinated by the S atoms forming the Cu–S–Sb framework,10–12,16 whereas the Ni2+ ions prefer to connect with amine ligands,9,20,21,24–26 this can also be seen in compound 2. In 2 the Ni2+ ion is completely coordinated by six N atoms of three dien ligands generating a [Ni(dien)2]2+ cation, further connection between Ni2+ ion and S atoms is prohibited. Thereby, the structure of compound 2 is similar to those of the copper-thioantimonates reported in the literature,10–12,16 showing a 2D layered structure. The layered structure of 2 is composed of large 18-membered Cu2Sb7S9 and small 6-membered CuSb2S3 hetero-rings. The large 18-membered Cu2Sb7S9 hetero-rings in this compound may be attributed to the large steric volume of cations and the strong electrostatic interaction between ions. It should be noted that in compound 1 there exist two types of bridging sulfur conformation, µ3–S and µ4–S, while in compound 2 there exists only µ2–S.
6. It should be noted that in amine-templated thioantimonates the Cu atoms tend to be coordinated by the S atoms forming the Cu–S–Sb framework,10–12,16 whereas the Ni2+ ions prefer to connect with amine ligands,9,20,21,24–26 this can also be seen in compound 2. In 2 the Ni2+ ion is completely coordinated by six N atoms of three dien ligands generating a [Ni(dien)2]2+ cation, further connection between Ni2+ ion and S atoms is prohibited. Thereby, the structure of compound 2 is similar to those of the copper-thioantimonates reported in the literature,10–12,16 showing a 2D layered structure. The layered structure of 2 is composed of large 18-membered Cu2Sb7S9 and small 6-membered CuSb2S3 hetero-rings. The large 18-membered Cu2Sb7S9 hetero-rings in this compound may be attributed to the large steric volume of cations and the strong electrostatic interaction between ions. It should be noted that in compound 1 there exist two types of bridging sulfur conformation, µ3–S and µ4–S, while in compound 2 there exists only µ2–S.
When secondary Sb–S bonds (the sum of the van der Waals radii of Sb and S is 3.8 Å 28) are considered, in compound 2 the Sb(1) and Sb(3) atoms have two (3.4970, 3.6514 Å) and one more S neighbors (3.7530 Å), respectively. No secondary Sb–S bonds are found in compound 1. In both title compounds, some nitrogen atoms are directed at anionic layers. N(1) and N(2) atoms in compound 1 both have two sulfur neighbours, while N(1), N(2) and N(5) atoms in compound 2 all have one S neighbour. The N⋯S distances (Table S2†) are in the range of 3.428(1)–3.586(1) Å, implying a possible presence of hydrogen bonding between the amines and the anionic framework.
In conclusion, compound 1 is the first example of a 3D inorganic–organic hybrid hetero-trimetallic sulfide. Compound 2 offers (CuSb3S62−)n anionic layers in which there exist large 18-membered Cu2Sb7S9 hetero-rings of about 8.1290(13) × 6.1292(10) Å, this is the largest pore in the amine-templated metal-thioantimonates reported so far. Owing to the different spatial structures and coordinating capability of the two organic amines, the as-synthesized two compounds show very varied structures. So it is reasonable to synthesize more compounds with novel structures through carefully choosing various organic amines.
Acknowledgements
This work was supported by grants from the 973 Program (2007CB815301 and 2006CB932904), the National Science Foundation of China (20333070, 20673118, 20871114), the Science Foundation of CAS (KJCX2-YW-M05) and of Fujian Province (2006J0014 and 2006F3132).Notes and references
- See examples: (a) N. F. Zheng, X. H. Bu, B. Wang and P. Y. Feng, Science, 2002, 298, 2366 CrossRef CAS; (b) F. Lips and S. Dehnen, Coord. Chem. Rev., 2009, 253, 1221 CrossRef.
- (a) D. Coucouvanis, A. Hadjikyriacou, M. Draganjac, M. G. Kanatzidis and O. Ileperuma, Polyhedron, 1986, 5, 349 CrossRef CAS; (b) M. R. Dubois, J. Am. Chem. Soc., 1983, 105, 3710 CrossRef; (c) M. Draganjac and T. B. Rauchfuss, Angew. Chem., Int. Ed. Engl., 1985, 24, 742 CrossRef; (d) M. D. Curtis, Appl. Organomet. Chem., 1992, 6, 429 CrossRef CAS.
- (a) R. Chevrel and M. Sergent, in Crystal Chemistry and Properties of Materials with Quasi-One-Dimensional Structures, ed. G. Rouxel, D. Reidel Publ. Co., Dordrecht, 1986, pp 315–373 Search PubMed; (b) T. Saito, W. Yamamoto, T. Yamagata and H. Tmoto, J. Am. Chem. Soc., 1988, 110, 1646 CrossRef CAS.
- (a) L. Chen and X. T. Wu, in Inorganic Chemistry in Focus II, ed. G. Meyer, D. Naumann and L. Weseman, Wiley, New York, 2005, 207 Search PubMed; (b) X. T. Wu, Q. Huang, Q. M. Wang, T. L. Sheng and J. X. Lu, in Transition Metal Sulfur Chemistry: Biological and Industrial Significance, ed. E. I. Stiefel and K. Matsumoto, ACS Symposium Series 653, American Chemical Society, Washington, DC, 1996, 282 Search PubMed.
- For example, see: (a) H. Yu, W. J. Zhang, X. T. Wu, T. L. Sheng, Q. M. Wang and P. Lin, Angew. Chem., Int. Ed., 1998, 37, 2520 CrossRef CAS; (b) Q. Huang, X. T. Wu, Q. M. Wang, T. L. Sheng and J. X. Lu, Angew. Chem., Int. Ed. Engl., 1996, 35, 868 CrossRef CAS; (c) Q. Huang, X. T. Wu, T. L. Sheng and Q. M. Wang, Inorg. Chem., 1995, 34, 4931 CrossRef CAS; (d) Q. Huang, X. T. Wu and J. X. Lu, Inorg. Chem., 1996, 35, 7445 CrossRef CAS; (e) Q. Huang, X. T. Wu and J. X. Lu, Chem. Commun., 1997, 703 RSC; (f) L. Chen, X. T. Wu, X. C. Gao, W. J. Zhang and P. Lin, J. Chem. Soc., Dalton Trans., 1999, 4303 RSC.
- M. Zhang, T. L. Sheng, X. H. Huang, R. B. Fu, X. Wang, S. M. Hu, S. C. Xiang and X. T. Wu, Eur. J. Inorg. Chem., 2007, 1606 CrossRef CAS.
- M. Schaefer, C. Näther and W. Bensch, Solid State Sci., 2003, 5, 1135 CrossRef CAS.
- R. Kiebach, W. Bensch, R.-D. Hoffmann and R. Z. Pöttgen, Z. Anorg. Allg. Chem., 2003, 629, 532 CrossRef CAS.
- R. Stähler and W. Bensch, Eur. J. Inorg. Chem., 2001, 3073 CrossRef CAS.
- A. V. Powell, R. Paniagua, P. Vaqueiro and A. M. Chippindale, Chem. Mater., 2002, 14, 1220 CrossRef CAS.
- A. V. Powell, S. Boissière and A. M. Chippindale, J. Chem. Soc., Dalton Trans., 2000, 4192 RSC.
- V. Spetzler, H. Rijnberk, C. Näther and W. Bensch, Z. Anorg. Allg. Chem., 2004, 630, 142 CrossRef CAS.
- P. Vaqueiro, A. M. Chippindale, A. R. Cowley and A. V. Powell, Inorg. Chem., 2003, 42, 7846 CrossRef CAS.
- V. Spetzler, C. Näther and W. Bensch, J. Solid State Chem., 2006, 179, 3541 CrossRef CAS.
- M. Schaefer, D. Kurowski, A. Pfitzner, C. Näther, Z. Rejai, K. Moller, N. Ziegler and W. Bensch, Inorg. Chem., 2006, 45, 3726 CrossRef CAS.
- V. Spetzler, C. Näther and W. Bensch, Inorg. Chem., 2005, 44, 5805 CrossRef CAS.
- M. Schaefer, R. Stähler, W. Kiebach, C. -R. Näther and W. Bensch, Z. Anorg. Allg. Chem., 2004, 630, 1816 CrossRef CAS.
- H. O. Stephan and M. G. Kanatzidis, J. Am. Chem. Soc., 1996, 118, 12226 CrossRef CAS.
- G. L. Schimek and J. W. Kolis, Chem. Mater., 1997, 9, 2776 CrossRef CAS.
- R. Stähler and W. Bensch, Z. Anorg. Allg. Chem., 2002, 628, 1657 CrossRef.
- H. O. Stephan and M. G. Kanatzidis, Inorg. Chem., 1997, 36, 6050 CrossRef CAS.
- R. Stähler, C. Näther and W. Bensch, Eur. J. Inorg. Chem., 2001, 1835 CrossRef CAS.
- X. Wang and F. Liebau, J. Solid State Chem., 1994, 111, 385 CrossRef CAS.
- R. Stähler, B. D. Mosel, H. Eckert and W. Bensch, Angew. Chem., Int. Ed., 2002, 41, 4487 CrossRef.
- R. Stähler, C. Näther and W. Bensch, J. Solid State Chem., 2003, 174, 264 CrossRef CAS.
- R. Kiebach, F. Studt, C. Näther and W. Bensch, Eur. J. Inorg. Chem., 2004, 2553 CrossRef CAS.
- (a) W. Bensch and M. Schur, Eur. J. Inorg. Chem., 1996, 33, 1149 Search PubMed; (b) M. Schur and W. Bensch, Z. Naturforsch., 2002, 57b, 1 Search PubMed; (c) W. Bensch, C. Näther and R. Stähler, Chem. Commun., 2001, 477 RSC; (d) R. Stähler, C. Näther and W. Bensch, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2001, 57, 26 CrossRef CAS; (e) A. V. Powell, S. Boissière and A. M. Chippindale, Chem. Mater., 2000, 12, 182 CrossRef CAS; (f) M. Schaefer, L. Engelke and W. Bensch, Z. Anorg. Allg. Chem., 2003, 629, 1912 CrossRef CAS; (g) R. Stähler and W. Bensch, J. Chem. Soc., Dalton Trans., 2001, 2518 RSC; (h) M. Schur, C. Näther and W. Bensch, Z. Naturforsch., B: Chem. Sci., 2001, 56, 79 CAS; (i) Y. Ko, K. Tan, J. B. Parise and A. Darovsky, Chem. Mater., 1996, 8, 493 CrossRef CAS; (j) J. B. Parise and Y. Ko, Chem. Mater., 1992, 4, 1446 CrossRef CAS; (k) M. Schur, A. Gruhl, C. Näther, I. Jess and W. Bensch, Z. Naturforsch., B: Chem. Sci., 1999, 54, 1524 CAS; (l) L. Engelke, C. Näther and W. Bensch, Eur. J. Inorg. Chem., 2002, 2936 CrossRef CAS; (m) M. Schur and W. Bensch, Eur. J. Solid State Inorg. Chem., 1997, 34, 457 CAS; (n) W. Bensch and M. Z. Schur, Z. Naturforsch., B: Chem. Sci., 1997, 52, 405 CAS; (o) A. Puls, M. Schaefer, C. Näther, W. Bensch, A. V. Powell, S. Boissière and A. M. Chippindale, J. Solid State Chem., 2005, 178, 1171 CrossRef CAS; (p) V. Spetzler, C. Näther and W. Bensch, Z. Naturforsch., B: Chem. Sci., 2006, 61, 715 CAS; (q) A. Puls, C. Näther and W. Bensch, Z. Anorg. Allg. Chem., 2006, 632, 1239 CrossRef CAS; (r) A. V. Powell, R. J. E. Lees and A. M. Chippindale, Inorg. Chem., 2006, 45, 4261 CrossRef CAS; (s) D. X. Jia, Q. X. Zhao, Y. Zhang, J. Dai and J. L. Zuo, Inorg. Chem., 2005, 44, 8861 CrossRef CAS; (t) R. J. E. Lees, A. V. Powell and A. M. Chippindale, Polyhedron, 2005, 24, 1941 CrossRef CAS; (u) D. X. Jia, Q. Y. Zhu, J. Dai, W. Lu and W. J. Guo, Inorg. Chem., 2005, 44, 819 CrossRef CAS; (v) V. Spetzler, R. Kiebach, C. Näther and W. Bensch, Z. Anorg. Allg. Chem., 2004, 630, 2398 CrossRef CAS.
- A. Bondi, J. Phys. Chem., 1964, 68, 441 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Selected distances and angles for 1 and 2 (Table S1); proposed hydrogen bonding geometry for 1 and 2 (Table S2). CCDC reference numbers 665818 for 1 and 665819 for 2. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b906640c |
‡ Crystal data, for 1: Mw = 896.99. monoclinic, space group P2(1)/n with a = 6.487(6)Å, b = 15.345(15)Å, c = 10.354(9)Å, β = 90.617(10)°, V = 1030.6(17) Å3. T = 293(2) K, Z = 2, Dcalcd = 2.891g cm−3, µ = 8.128 mm−1. 7832 reflections collected in the range 2.3736 < θ < 27.4835, 2336 unique reflections. R1 = 0.0917 and wR2 = 0.1318 for 1521 observed reflections. For 2: Mw = 886.21. Monoclinic, space group P2(1)/c with a = 17.107(4)Å, b = 9.850(2)Å, c = 14.530(3) Å, β = 103.559(4)°, V = 2380.2(9) Å3. T = 293(2) K, Z = 4, Dcalcd = 2.473g cm−3, µ = 5.551 mm−1. 17361 reflections collected in the range 2.40 < θ < 27.48, 5325 unique reflections. R1 = 0.0246 and wR2 = 0.0632 for 4836 observed reflections. Data for both compounds were corrected for Lorentz, polarization and absorption effects. The structures were solved and refined by full-matrix least-squares techniques on F2 using SHELXL-97 (Sheldrick, G. M. Universität Göttingen), all non-hydrogen atoms were refined anisotropically. Hydrogen atoms were generated geometrically and allowed to ride on their parent atoms with fixed isotropic displacement parameters. Both the metal ratios (Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sb = 1 Sb = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.19 4.19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.14 for 1, Ni 2.14 for 1, Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sb = 1 Sb = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.07 1.07![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.42 for 2) detected by energy dispersive spectroscopy are consistent with the theoretical stoichiometry (Ni 3.42 for 2) detected by energy dispersive spectroscopy are consistent with the theoretical stoichiometry (Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sb = 1 Sb = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for 1, Ni 2 for 1, Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sb = 1 Sb = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 for 2). 3 for 2). |
| This journal is © The Royal Society of Chemistry 2010 |
