Molecular tectonics: chaining cages into a 1-D coordination network†
Mei-Jin
Lin
,
Abdelaziz
Jouaiti
*,
Nathalie
Kyritsakas
and
Mir Wais
Hosseini
*
Laboratoire de Chimie de Coordination Organique (UMR 7140), Université de Strasbourg, Institut Le Bel, 4 rue Blaise Pascal, 67000, Strasbourg, France. E-mail: hosseini@chimie.u-strasbg.fr; Fax: +33 368851325; Tel: +33 368851323
First published on 4th November 2009
Abstract
The combination of ZnSiF6, a neutral infinite inorganic pillar, with a flexible organic tecton bearing at its both extremities a pyridyl moiety as a monodentate coordinating site, leads in the crystalline phase to the formation of a 1-D shashlik like coordination network composed of interconnected binuclear zinc macrotetracyclic cages.
The design and discovery of new coordination networks1 or metal–organic framework is of current interest because this class of solid state materials offers several applications in many areas such as storage,2 separation,3 and catalysis.4 Therefore, exploring different strategies to generate either known structures or unprecedentedly reported architectures, is pertinent and thus justified. In this area, the use of the molecular tectonics approach,5 centred on the design and combinations of molecular building blocks or tectons, is of special interest. In parallel, another design principle based on the concept of secondary building units (SBUs) was developed over the last ca two decades.6 However, so far both approaches are essentially centred on the design of individual tectons or SBUs as construction units.
Here, we report a conceptual extension by considering an infinite pillar as a tecton for the construction of new types of coordination networks.
The design of 1-D coordination networks, hybrid organic–metallic infinite periodic architectures possessing translational symmetry in one direction of space, may be based on combinations of an infinite construction unit, mainly an inorganic pillar offering in a periodic manner connecting sites (Fig. 1a), and acyclic organic tectons bearing at its each extremity a monodentate coordinating group. Depending on the nature (flexibility and curvature) of the spacer connecting the two coordinating sites, different types of 1-D architectures may be generated (Fig. 1b–e). The selection between these two categories, differing only in the type of connectivity, may be achieved through the design of the organic tecton. For a tecton possessing a proper curvature and thus capable of behaving like a chelate, one would expect the formation of a 1-D network resulting from the interconnection of metallamacrobicycles along the pillar (Fig. 1b). For tectons, unable to bite on the same metal centre, the formation of the three different architectures (Fig. 1c–e) may be envisaged. The case schematically presented in Fig. 1e is interesting since it consists of a rod composed of interconnected metallamacrotetracyclic cages. Although many examples of discrete metallamacrocycles have been reported,7 only few cases of networks based on molecular cages have been documented.8
 | ||
| Fig. 1 Schematic representation of a pillar (a) and different 1-D networks based on the formation of bicycles (b–d) and a tetracycle (e) resulting from the combination of ZnSiF6 with curved organic tectons. | ||
As for the inorganic pillar, SiF62− anion is the candidate of choice. Indeed, it is known that the combination of SiF62− anion with divalent Zn, Co and Cu cations leads in the presence of organic ligands to the formation of MSiF6 pillars resulting from the bridging of consecutive metal centres by the anion through the two fluorine atoms occupying the axial positions (Fig. 1a).9 We have previously used ZnSiF6 for the design and generation of 2-D tubular10a and 3-D cuboid10b types of networks.
As flexible organic tecton bearing two monodentate coordinating sites at their each end, compounds 1–4 (Scheme) were designed and prepared. The choice of these tectons was motivated by the previous observation on the formation of a discrete binuclear coordination cage 1, {[Zn(1)2(H2O)2](ClO4)2}2 with a Zn–Zn distance of 8.1 Å,11 which should fit the predefined distance of ca 7.5 Å between consecutive Zn2+ cations in the ZnSiF6 pillar. Tectons 1–4 differ only by the nature of the spacer connecting the two 4-thiapyridyl units. Whereas for tectons 1 and 2, the spacer is a hydrocarbon chain, for the other two, the spacer is either (CH2)2-O-(CH2)2 or (CH2)2-S-(CH2)2 fragment. In all four cases, the junction between 4-thiapyridyl units is of the thioether type. The synthesis of 1 had been previously reported11 and for the other three compounds, it was straightforward.‡
 | ||
| Scheme 1 | ||
Tectons 1–4 were obtained in 58, 62, 67 and 70% yields, respectively, upon condensation in refluxing CH3CN of 4-mercaptopyridine 9 with compounds 5–8, respectively, in the presence of K2CO3.
In crystallization tubes, upon slow diffusion of an EtOH solution of ZnSiF6·6H2O into a CHCl3 solution of tecton 1–4 through a buffer layer of DMSO, colourless crystalline materials were obtained after several days. Unfortunately, only in the case of 1 were obtained suitable crystals for structural studies by X-ray diffraction on single-crystal. In all other cases, despite many attempts and changes in the crystallisation conditions, poor quality polycrystalline materials were obtained. The structural investigation revealed that the crystal (tetragonal, space group I4/m) was composed of the organic tecton 1, ZnSiF6 and EtOH solvent molecules.§ As expected from the design of 1 and the use of ZnSiF6, the latter component indeed forms an inorganic pillar (Fig. 1a) and the organic tecton 1, by bridging two neighbouring Zn2+ cations, leads to the formation of binuclear metallamacrotetracycles along the pillar axis (Fig. 1e). The resulting structure is a neutral 1-D “shashlik” like coordination network (Fig. 2a) which may also be described as [Zn2(1)4]4+ cationic cavities encapsulating SiF62− anions ([Zn2(1)4-SiF6]2+) interconnected by SiF62− anions.
 | ||
| Fig. 2 Portions of X-ray structures of the 1-D shashlik like coordination network obtained upon combining 1 and ZnSiF6 viewed perpendicular to the network axis (a), the H-bonding pattern between the encapsulated SiF62− anion and the interior of the cage (b) and along the direction of ZnSiF6 pillar showing the eclipsed configuration for the cage and staggered arrangement for consecutive anions (c). For clarity, solvent molecules are omitted. | ||
The inorganic pillar is formed by two types of SiF62− anions, one inside the cage and the other bridging consecutive cages.
The anion located within the cationic cage interacts with the interior of the cavity by both coordination and hydrogen bonding. The SiF62− anion bridges the two Zn2+ cations composing the metallamacrotetracycle through rather weak Zn–F bonds involving the two axial F atoms (dZn–F = 2.47 Å). For the other four F atoms occupying the square base of the octahedron, the Zn–F distance is 1.62 Å. The encapsulated SiF62− anion forms also rather strong H–F hydrogen bonds (Fig. 2b) with some of the H atoms belonging to the four organic tectons 1 surrounding the cage (dF–H in the range of 2.45–2.47 Å). The SiF62− anion located outside the cavity, connects consecutive cages along the c axis through again Zn–F bonds with a dZn–F distance of 2.07 Å which is comparable to the one previously observed.10b Consecutive SiF62− anions adopt a staggered arrangement with an angle of 42.5° (Fig. 2c).
The zinc cation adopts a slightly distorted octahedral geometry with its coordination sphere composed of two F and four N atoms (Fig. 2a). The N–Zn–N and F–Zn–N angles are in the range of 87.7 to 92.3° deviating only slightly (2.3°) from 90°. The two F atoms belonging to either the encapsulated or external SiF62− anions involved in the formation of the pillar occupy the apical positions. The four N atoms belonging to four tectons 1 occupy the equatorial positions (dZn–N = 2.11 Å). Two different Zn–Zn distances, one short (7.6 Å) corresponding to the distance between cages and one longer (8.4 Å) corresponding to the distance between the two metal centres composing the cage are observed. Both distances are comparable to those of 7.6 and 8.1 Å previously obtained for 3-D cuboid architectures based on ZnSiF610b and for a discrete cage type structure generated upon combining tectons 1 with Zn(ClO4)2, respectively.11 Along the pillar, consecutive cages are eclipsed (Fig. 2c).
In dealing with packing, 1-D networks are arranged in parallel fashion and slipped by ca 7.6 Å (Fig. 3). Owing to the separation and partial filling of voids by encapsulated SiF62− anions, four rather small cavities around each cage are obtained. They are occupied by ethanol molecules.
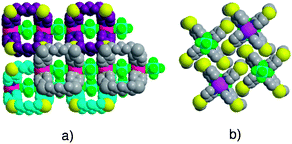 | ||
| Fig. 3 Packing of 1·ZnSiF6 1-D networks viewed perpendicular (a) and along direction of ZnSiF6 pillar. For clarity, the adjacent chains are differentiated by colour. | ||
The purity of the crystalline material was investigated by X-ray diffraction on powder (Fig. 4). The latter technique revealed the presence of a pure phase, i.e. almost perfect matching between observed and simulated patterns.
 | ||
| Fig. 4 Comparison of the simulated (a) and recorded (b) PXRD patterns for 1·ZnSiF6. | ||
The thermal stability of 1·ZnSiF6 was investigated by TGA under nitrogen atmosphere (Fig. 5). The first loss of mass corresponding to the release of EtOH molecules (ca 15%) occurred between 100 and 150 °C. The higher observed temperature than the boiling point of ethanol is attributed to the close nature of the cavities and the presence of hydrogen bond-type interactions between the networks and solvent molecules (dOH–F = 1.93 Å and dCH–F = 2.49 Å). The decomposition of the sample appeared at ca. 230 °C.
 | ||
| Fig. 5 TGA patterns for 1·ZnSiF6 under N2 flow with 5 °C min−1 scan rate. | ||
In conclusion, we have demonstrated that for the design and generation of 1-D coordination networks, instead of considering molecular tectons, i.e. discrete molecules as building blocks, one may use an infinite inorganic pillar based on ZnSiF6 as a periodic rod-type construction unit with imposed distance between consecutive square planar nodes. The combination of such unit with an organic tecton bearing at its each extremity a monodentate coordinating site and possessing the appropriate curvature, leads to the formation of a 1-D network based on the interconnection of binuclear zinc metallamacrotetracyclic cages. Currently we are investigating the extension of this strategy to the design and formation of other periodic architectures using other organic tectons and metal centres.
Acknowledgements
We thank the Université de Strasbourg, the Institut Universitaire de France, the Ministry of Education and Research, the CNRS and Marie Curie Est Actions FUMASSEC Network (Contrat N° MEST-CT-2005-020992) for financial support.Notes and references
- (a) B. F. Abrahams, B. F. Hoskins and R. Robson, J. Am. Chem. Soc., 1991, 113, 3606 CrossRef CAS; (b) A. J. Blake, N. R. Champness, P. Hubberstey, W.-S. Li, M. A. Withersby and M. Schröder, Coord. Chem. Rev., 1999, 183, 117 CrossRef CAS; (c) B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629 CrossRef CAS; (d) S. Kitagawa, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef CAS; (e) G. Férey, Chem. Soc. Rev., 2008, 37, 191 RSC.
- (a) J. L. C. Rowsell, E. C. Spencer, J. Eckert, J. A. K. Howard and O. M. Yaghi, Science, 2005, 309, 1350 CrossRef CAS; (b) X. Zhao, B. Xiao, A. J. Fletcher, K. M. Thomas, D. Bradshaw and M. J. Rosseinsky, Science, 2004, 306, 1012 CrossRef CAS.
- (a) S. M. Kuznicki, V. A. Bell, S. Nair, H. W. Hillhouse, R. M. Jacubinas, C. M. Braunbarth, B. H. Toby and M. Tsapatsis, Nature, 2001, 412, 720 CrossRef CAS; (b) M. E. Kosal, J.-H. Chou, S. R. Wilson and K. S. Suslick, Nat. Mater., 2002, 1, 118 CrossRef CAS; (c) C. Jiang, A. Lesbani, R. Kawamoto, S. Uchida and N. Mizuno, J. Am. Chem. Soc., 2006, 128, 14240 CrossRef CAS.
- (a) O. Ohmori and M. Fujita, Chem. Commun., 2004, 1586 RSC; (b) J. S. Seo, D. Whang, H. Lee, S. I. Jun, J. Oh, Y. J. Jeon and K. Kim, Nature, 2000, 404, 982 CrossRef CAS; (c) C.-D. Wu, A. Hu, L. Zhang and W. Lin, J. Am. Chem. Soc., 2005, 127, 8940 CrossRef CAS.
- (a) M. Simard, D. Su and J. D. Wuest, J. Am. Chem. Soc., 1991, 113, 4696 CrossRef; (b) S. Mann, Nature, 1993, 365, 499 CrossRef CAS; (c) M. W. Hosseini, Acc. Chem. Res., 2005, 38, 313 CrossRef CAS; (d) M. W. Hosseini, CrystEngComm, 2004, 6, 318 RSC.
- (a) O. M. Yaghi, M. O'Keeffe, N. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705 CrossRef CAS.
- (a) P. J. Stang and B. Olenyuk, Acc. Chem. Res., 1997, 30, 502 CrossRef CAS; (b) K. Suzuki, M. Kawano and M. Fujita, Angew. Chem., Int. Ed., 2007, 46, 2819 CrossRef CAS; (c) D. L. Caulder and K. N. Raymond, Acc. Chem. Res., 1999, 32, 975 CrossRef CAS; (d) G. F. Swiegers and T. J. Malefetse, Chem. Rev., 2000, 100, 3483 CrossRef CAS; (e) S. Sato, Y. Ishido and M. Fujita, J. Am. Chem. Soc., 2009, 131, 6064 CrossRef CAS; (f) C. Y. Su, Y. P. Cai, C. L. Chen, M. D. Smith, W. Kaim and H. C. Loye, J. Am. Chem. Soc., 2003, 125, 8595 CrossRef CAS.
- (a) J. Park, S. Hong, D. Moon, M. Park, K. Lee, S. Kang, Y. Zou, R. P. John, G. H. Kim and M. S. Lah, Inorg. Chem., 2007, 46, 10208 CrossRef CAS; (b) R.-Q. Zou, L. Jiang, H. Senoh, N. Takeichi and Q. Xu, Chem. Commun., 2005, 3526 RSC.
- (a) R. A. J. Driessen, F. B. Hulsbergen, W. J. Vermin and J. Reedijk, Inorg. Chem., 1982, 21, 3594 CrossRef CAS; (b) L. R. MacGillivray, S. Subramanian and M. J. Zaworotko, J. Chem. Soc., Chem. Commun., 1994, 1325 RSC; (c) S.-I. Noro, S. Kitagawa, M. Kondo and K. Seki, Angew. Chem., Int. Ed., 2000, 39, 2081 CrossRef; (d) M.-C. Suen and J.-C. Wang, Struct. Chem., 2006, 17, 315 CrossRef CAS; (e) M.-C. Suen, Z.-K. Chan, J.-D. Chen, J.-C. Wang and C. H. Hung, Polyhedron, 2006, 25, 2325 CrossRef CAS.
- (a) M. J. Lin, A. Jouaiti, D. Pocic, N. Kyritsakas, J. M. Planeix and M. W. Hosseini, submitted (b) M. J. Lin, A. Jouaiti, N. Kyritsakas and M. W. Hosseini, CrystEngComm, 2009, 11, 189 RSC.
- Y. B. Xie, J. R. Li, C. Zhang and X. H. Bu, Cryst. Growth Des., 2005, 5, 1743 CrossRef CAS.
- G. M. Sheldrick. Program for the Solution of Crystal Structures, University of Gottingen, Gottingen, Germany, 1997 Search PubMed.
Footnotes |
| † CCDC reference number 746134. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b917864c |
| ‡ General method for the synthesis of compounds2–4: Under argon and at room temperature, 4-mercaptopyridine 9 (1.22 g, 11 mmol) and K2CO3 in excess (4.64 g, 33.4 mmol) were dissolved in CH3CN (50 mL). To the stirred mixture, 5 mmol of 6 or 7 or 8 was added. The solution was heated to reflux overnight. The mixture was allowed to cool to rt and was filtered. The solvent was removed under reduced pressure and the mixture purified by column chromatography (SiO2, CH2Cl2). The pure final products were obtained as yellowish oils. Tecton 2 (0.94 g, 62%): 1H-NMR (300 MHz, 298 K, CDCl3): δ [ppm] = 0.95–0.96 (m, 3H), 1.65–1.75 (m, 5H), 2.94–2.97 (m, 4H), 7.47–7.59 (dd, 4H, J = 3, 1.5), 8.64–8.66 (dd, 4H, J = 3, 1.8); 13C-NMR (75 MHz, 298 K, CDCl3): δ [ppm] = 22.6, 31.3, 33.5, 34.1, 120.4, 149.3. Tecton 3 (0.98 g, 67%): 1H-NMR (300 MHz, 298 K, DMSO-d6): δ [ppm] = 3.23–3.27 (m, 4H), 3.67–3.71 (m, 4H), 7.27–7.29 (dd, 4H, J = 3, 1.8), 8.34–8.36 (dd, 4H, J = 3, 1.5); 13C-NMR (75 MHz, 298 K, DMSO-d6): δ [ppm] = 40.2, 68.7, 121.0, 148.6, 149.6. Tecton 4 (1.08 g, 70%): 1H-NMR (300 MHz, 298 K, CDCl3): δ [ppm] = 3.13–3.25 (m, 4H), 3.72–3.74(m, 4H), 7.26–7.29 (dd, 4H, J = 3.6, 2.4), 8.37–8.40 (dd, 4H, J = 3.6, 1.5); 13C-NMR (75 MHz, 298 K, CDCl3): δ [ppm] = 33.2, 40.1, 121.2, 149.4, 149.6. |
| § Crystallography: Data were collected at 173(2) K on a Bruker SMART CCD diffractometer with Mo Kα radiation. The structures were solved using SHELXS-9712 and refined by full matrix least-squares on F2 using SHELXL-97 with anisotropic thermal parameters for all non H atoms. The latter were introduced at calculated positions and not refined (riding model). Crystal data for 1·ZnSiF6: 2(C30H36F6N4S4SiZn)·2CH3CH2OH, M = 880.46, tetragonal, space group I4/m, a = b = 15.930(2), c = 15.941(4) Å, V = 4045.3(12) Å3, T = 173(2) K, Z = 4, Dc = 1.446 g cm−3, μ = 0.907 mm−1, 10010 collected reflections, 2417 independent (Rint = 0.0644), GooF = 1.002, R1 = 0.0491, wR2 = 0.1194 for I > 2σ(I) and R1 = 0.1147, wR2 = 0.1495 for all data. |
| This journal is © The Royal Society of Chemistry 2010 |
