High-precision analysis of Sr/Ca and Mg/Ca ratios in corals by laser ablation inductively coupled plasma optical emission spectrometry
Wenfeng
Deng
a,
Ying
Liu
a,
Gangjian
Wei
*ab,
Xianhua
Li
a,
Xianglin
Tu
a,
Luhua
Xie
a,
Hong
Zhang
a and
Weidong
Sun
*ac
aCAS Key Laboratory of Isotope Geochronology and Geochemistry, Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, Guangzhou, 510640, China. E-mail: gjwei@gig.ac.cn; Fax: +86-20-85290093; Tel: +86-20-85290093
bCAS Key Laboratory of Marginal Sea Geology, Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, Guangzhou, 510640, China
cResearch Center for Mineral Resources, School of Earth and Space Sciences, University of Science and Technology of China, Hefei, 230026, China. E-mail: weidongsun@gig.ac.cn; Fax: +86-20-85291510; Tel: +86-20-85290215
First published on 14th October 2009
Abstract
A method has been developed to determine high-precision Sr/Ca and Mg/Ca ratios in corals by laser ablation inductively coupled plasma optical emission spectrometry (LA-ICP-OES) using aqueous solution standard calibration. Simultaneous determination of the signals of the entire analytical wavelengths by ICP-OES and the high performance of the new type of LA system (Resonetics 193 nm ArF excimer laser-ablation system, RESOlution M-50) improve the precision for elemental ratios. Repeated measurements on a coral base synthesized working standard, BH-7, provide precisions of about 0.4% and 0.8% for the Sr/Ca and Mg/Ca ratios, respectively, which are better than the formerly reported precision by the LA-ICP-MS method, about 1%. Such precision is comparable to those obtained by the solution nebulization-ICP-OES (SN-ICP-OES) method, and is adequate for paleoclimate reconstruction. In addition, the LA-ICP-OES can provide results with much higher spatial/time resolution. Comparisons between the LA and SN methods were handled by measuring along the same track of a coral. The Sr/Ca results by these two methods agree quite well with each other. The LA-ICP-OES method is very promising for the analysis of element/Ca ratios in coral and other carbonates used in paleoclimate studies such as stalagmite. Systematic discrepancy, however, was observed in the Mg/Ca ratios, likely due to the existing state of magnesium in the coral skeleton.
Introduction
Corals secrete a calcareous skeleton of aragonite (CaCO3) into which some trace elements such as Sr and Mg appear to be incorporated as a function of seawater temperature. Thus, Sr/Ca and Mg/Ca ratios in fossil corals can be used to reconstruct past sea surface temperature (SST).1–3 Precise determination of Sr/Ca and Mg/Ca ratios is the prerequisite to obtain accurate paleo-SST records from fossil corals. Generally, Sr/Ca, Mg/Ca and other element/Ca ratios in corals are measured by isotope dilution thermal ionization mass spectrometry (ID-TIMS) and isotope dilution inductively coupled plasma mass spectrometry (ID-ICP-MS) in previous studies.1,4–6 More recently, the inductively coupled plasma optical emission spectrometry (ICP-OES) method, which can measure element/Ca ratios in carbonate more rapidly and more precisely, has been widely used in paleoclimatic studies.2,7–10 This has the advantages of employing a less expensive instrument compared to ICP-MS and TIMS technologies, with reduced costs and minimal sample preparation.7All above analytical methods need off-line chemical pre-treatments using variable acids and considerable amounts of sample. This is also time consuming. The laser ablation (LA) sampling technique can overcome these shortcomings, which requires little sample preparation and allows rapid analysis of solid-state samples at high spatial resolutions up to 20 µm. The LA-ICP-MS technique has been adopted to determine element/Ca ratios and trace element concentrations in corals.11,12 However, element/Ca ratios obtained by LA-ICP-MS generally have poor precision, and are difficult to be used to quantitatively reconstruct paleoclimatic records such as SST. Compared to the dynamic scan approach on data acquisition of ICP-MS, ICP-OES equipped with a Charge Coupled Device (CCD) detector has the advantage of simultaneously determining different analytical wavelengths. This can eliminate the errors from signal fluctuation induced by instability of the ICP source to a certain extent, and achieve a higher precision than ICP-MS. Here, we establish a method to obtain high-precision Sr/Ca and Mg/Ca ratios in corals by LA-ICP-OES, which can be used in quantitatively reconstructing paleoclimatic records.
Experimental
Coral sample preparation
The analyzed coral is a modern Porites lutea, BH-2, collected from the Weizhou Island, northern South China Sea in 2007. The sample was cut into 5 mm thick, 27 mm wide and 50 mm long slabs along the major growth axis using a high-speed diamond saw. This shape is designed to fit the sample holder of the RESOlution M-50 laser ablation system. Then, the coral slabs were rinsed by a set of chemical procedures as described by Wei et al. (2007).10A coral base working standard, BH-7, was synthesized by mixing coral powder, SiO2 and Li2B4O7 at a ratio of 1:1:1, and fused into a glass disc, which is similar to the method described by Sinclair et al. (1998).12 It was repeatedly measured along with the samples to monitor the reproducibility of the measurements.
Instrumentation
A Varian Vista Pro whole spectrum direct-reading ICP-OES was employed in this study. This instrument equipped with a solid state CCD detector allowing the simultaneous monitoring of all the analytical lines, and an optimized optical design gives excellent signal-to-noise performance, resulting in low detection limits. It covers a wide wavelength range (167–785 nm) with good stability and sensitivity for emission lines.A Resonetics 193 nm ArF excimer laser-ablation system (RESOlution M-50) was connected to the ICP-OES. This system has a state-of-the-art 50 × 50 mm sample cell developed by Laurin Technic (Canberra Australia), which responds quickly to changes in the sample or ablation conditions (99% signal washout in less than 1.5 seconds) and can give a smooth signal with laser pulse rates down to 1 Hz using the “squid” smoothing device for optimal depth profiling.13 The sensitivity and fractionation are independent of the sampling position in the cell.
The sample aerosol produced by laser ablation was transported by a tube drilled through the waste liquid tube of the spray chamber of the ICP-OES (Fig. 1), and it was first mixed with the injected solution through the intrinsic concentric nebulizer of the ICP-OES, and then was introduced into the ICP source (Fig. 1). This design allows dual-inlet introduction of aerosols without additional modifications on the spray chamber. The advantages of the solution introduction are as follows. One is to maintain the stability of the plasma, because the plasma will ignite off without solution introduction for our ICP-OES. Consistent with previous study, this method can produce more stable and more robust conditions than dry plasma conditions.14 The other is to introduce solution standards as external calibration standards when no laser produced aerosol was introduced (laser off).
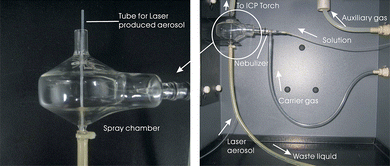 | ||
| Fig. 1 Diagram of the sample introduction system for solution and laser produced aerosols. The left image shows the tube for the laser produced aerosol drills through the waste liquid tube of the spray chamber of the ICP-OES. The right image shows the full structure of the dual-inlet introduction design for solutions and laser aerosols. | ||
An automated gas-handling system with mass flow controllers supplies He gas to the LA cell. Ablation in He substantially reduces the deposition of ablated material onto the sample surface and greatly increases signal intensities.15 The He gas and the sample aerosol flow to the spray chamber of the ICP-OES via the “squid” and then are mixed with the solution injected by Ar carrier gas, so that the He and Ar flow for each sample stream was kept constant as various combinations of sample were introduced, including solid sampling (continuous ablation with 3% HNO3 solution blank nebulization), solution (laser stopped with solution standard nebulization), and background acquisition (laser stopped with 3% HNO3 solution blank nebulization).16 This introduction mode can maintain the “wet” plasma during the whole analysis procedure, which can eliminate the difference between “dry” and “wet” plasmas when nebulized aqueous standards were used for calibration,17 and help to diminish the matrix difference between aerosols samples and aqueous standards.16
Standard calibration strategies
For LA-ICP-OES/MS analysis, external standard calibration is generally adopted to obtain quantitative results of the unknown samples. An ideal external standard should be chemically and physically matched to the unknown samples.18–20 However, commercial carbonate matrix standards for LA-ICP-OES/MS analysis are not available currently. Perkins et al. (1991)20 used pressed carbonate powder for calibration on quantitative analysis of trace elements in carbonates by LA-ICP-MS. It is however very difficult to produce homogeneous standards by mixing the powder of carbonates.20 Alternatively, CaSiO3 glass standard synthesized from coral powder is generally used as external standards for the analysis of elements/Ca ratios in corals by LA-ICP-MS,12 but the accurate concentrations of elements of such glass standards need to be further verified.11 Compared with the above carbonate base solid standards, aqueous solution standards have more accurate concentration values, and are homogeneous. The problem is that it does not match the matrix of the aerosols samples. Thompson et al. (1989) developed a method using an aqueous solution as external standard, in which a dual-inlet device, one for the aerosol samples and one for the aqueous solution, was adopted, and successfully applied in element concentration measurements using LA-ICP-OES.21 This was further applied by Cromwell and Arrowsmith (1995), in which mixed-sample introduction was adopted.16 The aerosols ablated by laser were first mixed with the nebulized aqueous solution before they were transported to the ICP. This can provide similar ICP conditions both for the solid samples and aqueous standards, and achieve reliable results for solid samples using aqueous solutions as external standards.16Herein, we use aqueous solutions as external standards for calibration, and the mixed-sample introduction method is adopted in precise measurements of element/Ca ratios in corals. The standard solution was an aqueous mixture of Ca, Sr and Mg, with the respective concentrations very similar to corals. This further diminishes the matrix difference between the aqueous solution standard and the coral samples, favorable for more precise element/Ca results.
Method development
The laser output beam is set to 80 µm wide in parallel to the growth axis of the coral and 600 µm long in perpendicular to the growth axis on the coral surface by a rectangular variable aperture supplied by Resonetics. Ablation points along a path mode was adopted, in which the signals of single points rather than continuous instant scan signals are acquired by ICP-OES with a spatial resolution of about 80 µm. This ablation mode is different from the scan mode adopted by the LA-ICP-MS method,11,12 and the data of a single point can be distinguished. The working conditions of the laser and ICP-OES are summarized in Table 1. Prior to data acquisition, the synthesized standard glass and the coral were pre-ablated to clean the surface of contaminants.| ICP-OES: Varian Vista Pro | |
| Power | 1200 W |
| Plasma gas flow | 15 L/min |
| Auxiliary gas flow | 1.5 L/min |
| Nebulizer pressure | 200 kPa |
| Integration time | 1 s |
| Laser-ablation system: Resonetics RESOlution M-50 | |
| He gas flow | 0.4 L/min |
| Laser repetition rate | 10 Hz |
| Laser spot size | 80 µm × 600 µm |
| Ablation mode | points along a path |
Ca, Sr and Mg were detected on 318.127 nm, 407.771 nm and 279.553 nm spectral lines, respectively. The whole procedure to measure a sample contains 8 replicated measurements, and the integration time for each measurement was 1 s. Longer integration times can help to diminish shot noise of the CCD detector and achieve better precision,22 and the 1 s integration time appeared to reach good precision for Sr, Ca and Mg based on our routine measurement on the ICP-OES employed here. Prior to the inlet of laser ablated aerosols, a mixed Ca, Sr and Mg standard solution was injected through the intrinsic concentric nebulizer and measured to calculate the concentrations of Ca, Sr and Mg relative to intensities. During the measurement with laser ablated aerosols injected, intensity ratios of Sr/Ca and Mg/Ca rather than the intensity of a single line were collected simultaneously, to eliminate the influence of short term fluctuations in intensities. Former studies suggested that by using internal standardization an excellent precision of the LA-ICP-OES analysis method can be obtained.23,24 The simultaneously collecting of Sr/Ca and Mg/Ca intensity ratios in this study resembles the use of Ca as an internal standard. Therefore, high precisions are expected for Sr/Ca and Mg/Ca ratios. The linear instrument drift on Sr/Ca and Mg/Ca ratios is corrected by the approach used for analysis of gas-source stable isotopes, i.e. the sandwich style measurement of standard-sample-standard suggested by Schrag (1999).7 In order to increase the efficiency of the measurement, we repeatedly measured the synthesized coral base glass standard, BH-7, after every five measurements of the samples. The measured Sr/Ca and Mg/Ca ratios of BH-7 were calibrated to their long-term averages to correct the machine drift assuming that the machine drift between the five measurements was linear, as details described by Wei et al. (2007).10
Results and discussion
The precision of this LA-ICP-OES method has been estimated from the reproducibility of the results of the synthesized glass standard. 60 measurements of BH-7 over a 10-hour time span revealed precisions (relative standard deviation, 1σ) of 0.4% for Sr/Ca and 0.8% for Mg/Ca (Fig. 2), respectively, which are better than those obtained by LA-ICP-MS.11,12 These precisions meet the requirement of less than ± 0.5 °C temperature error range for SST reconstruction by coral Sr/Ca and Mg/Ca ratios. This good precision may mainly benefit from the simultaneous determination of all analytical wavelengths of ICP-OES and the high performance of the new type of LA system employed here. The precision is only slightly poorer than those by the solution nebulization method but is adequate for paleoclimate reconstruction.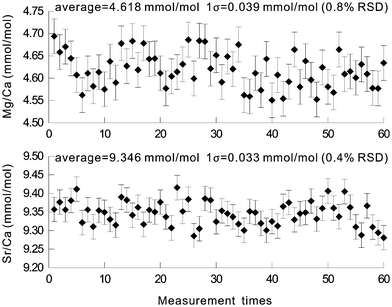 | ||
| Fig. 2 Precisions of the Sr/Ca and Mg/Ca ratios in the glass standard synthesized from coral powder. The upper graph is for the Mg/Ca ratio and the lower graph for the Sr/Ca ratio. | ||
Fig. 3 shows the Sr/Ca and Mg/Ca ratios of a coral measured by this method along a main growth axis in ∼80 µm spatial resolution, and the results measured using the solution nebulization ICP-OES method along the same axis in ∼1 mm spatial resolution are also shown for comparison (Fig. 3). The high-resolution Sr/Ca and Mg/Ca profiles by LA-ICP-OES appear to be noisier than the solution nebulization measured profiles (Fig. 3). Because of the heterogeneous fine-scale composition and structure of the coral, LA-ICP-OES may have caught daily SST variations. To evaluate the accuracy of this method, we filtered the LA-ICP-OES data by 11 point moving average, and then compared them with the results by the solution nebulization ICP-OES method. This accuracy evaluation method may be useful in the absence of international standards for laser ablation measurement on corals. The averages and the variation ranges of these results are listed in Table 2. The average of the Sr/Ca ratios by LA-ICP-OES is close to that by SN-ICP-OES with a discrepancy of 2%. This difference is within the analytical error range. The variation range of the Sr/Ca ratios by LA-ICP-OES is much lager than that by SN-ICP-OES, which may be attributed to the heterogeneous fine-scale composition structure in corals and a daily SST variation. After filtering by an 11 point moving average, which yields similar spatial resolution to that of the SN-ICP-OES method, the Sr/Ca variation ranges by these two methods are very close (Fig. 3). In particular, the variation pattern of the filtered Sr/Ca ratios by LA-ICP-OES matches that by SN-ICP-OES very well (Fig. 3).
| Element/Ca | LA-ICP-OES analysis | SN-ICP-OES analysis | ||
|---|---|---|---|---|
| Average | Variation range (data after filtering) | Average | Variation range | |
| Sr/Ca (mmol/mol) | 9.004 | 8.63–9.59 | 8.818 | 8.55–9.37 |
| Mg/Ca (mmol/mol) | 3.639 | 2.86–4.31 | 4.649 | 3.98–6.01 |
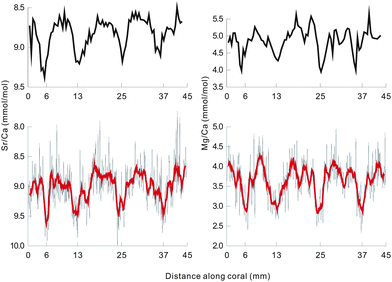 | ||
| Fig. 3 Comparisons of Sr/Ca and Mg/Ca ratios in BH-2 coral obtained by LA-ICP-OES and SN-ICP-OES. The two upper graphs show the results of SN-ICP-OES, and the two lower graphs show those of LA-ICP-OES. The bold lines in the lower two represent the data of LA-ICP-OES filtered by an 11 point moving average. | ||
Unlike the Sr/Ca ratios, the Mg/Ca results by these two methods are significantly different both in the averages and the variation ranges, with a relative deviation between the two averages of up to 20%. This may be attributed to the heterogeneous storage of magnesium in coral aragonite. A previous study indicates that 10–30% of the magnesium is adsorbed on the coral skeletal surface rather than in the aragonite crystal,25 and these adsorptive magnesium can be easily removed by chemical cleaning.26 The LA method can only measure several microns of the coral surface. Thus, lower Mg/Ca ratios were obtained than those by the SN method, which are measured on bulk samples. Despite the difference on values, the variation pattern of the filtered Mg/Ca ratios by the LA method is also very similar to that by the SN method, comparable to the Sr/Ca ratios (Fig. 3). This demonstrates that the LA-ICP-OES method is very promising for element/Ca ratio analyses in corals and other carbonates, such as stalagmite, that are popularly used in paleoclimate studies.
Acknowledgements
Critical comments and constructive suggestions by Dr May Copsey and two anonymous referees are highly appreciated. This work was supported by the Knowledge Innovation Program of the Chinese Academy of Sciences (KZCX2-YW-138, GIGCX-08-03). This is contribution No. IS-1106 from GIGCAS.References
- J. W. Beck, R. L. Edwards, E. Ito, F. W. Taylor, J. Recy, F. Rougerie, P. Joannot and C. Henin, Science, 1992, 257, 644–647.
- T. Mitsuguchi, E. Matsumoto, O. Abe, T. Uchida and P. J. Isdale, Science, 1996, 274, 961–963 CrossRef.
- S. V. Smith, R. W. Buddemeier, R. C. Redalje and J. E. Houck, Science, 1979, 204, 404–407 CrossRef CAS.
- D. W. Lea and P. A. Martin, Geochim. Cosmochim. Acta, 1996, 60, 3143–3149 CrossRef CAS.
- F. LeCornec and T. Correge, J. Anal. At. Spectrom., 1997, 12, 969–973 RSC.
- G. J. Wei, M. Sun, X. H. Li and B. F. Nie, Palaeogeogr., Palaeoclimatol., Palaeoecol,, 2000, 162, 59–74 CrossRef.
- D. P. Schrag, Paleoceanography, 1999, 14, 97–102 CrossRef.
- Y. L. Sun and M. Sun, Appl. Spectrosc., 2003, 57, 711–714 CrossRef CAS.
- G. J. Wei, W. F. Deng, Y. Liu and X. H. Li, Palaeogeogr., Palaeoclimatol., Palaeoecol,, 2007, 250, 126–138 CrossRef.
- G. J. Wei, W. F. Deng, K. F. Yu, X. H. Li, J. X. Zhao and W. D. Sun, Paleoceanography, 2007, 22, PA3206, DOI:10.1029/2006PA001270.
- S. J. Fallon, M. T. McCulloch, R. van Woesik and D. J. Sinclair, Earth Planet. Sci. Lett., 1999, 172, 221–238 CrossRef CAS.
- D. J. Sinclair, L. P. J. Kinsley and M. T. McCulloch, Geochim. Cosmochim. Acta, 1998, 62, 1889–1901 CrossRef CAS.
- W. Muller, M. Shelley, P. Miller and S. Broude, J. Anal. At. Spectrom., 2009, 24, 209–214 RSC.
- C. Dubois, N. Gilon, C. P. Lienemann, S. Morin and J. M. Mermet, J. Anal. At. Spectrom., 2005, 20, 950–953 RSC.
- S. M. Eggins, L. P. J. Kinsley and J. M. G. Shelley, Appl. Surf. Sci., 1998, 127, 278–286 CrossRef.
- E. F. Cromwell and P. Arrowsmith, Anal. Chem., 1995, 67, 131–138 CrossRef CAS.
- R. E. Russo, X. L. Mao, H. C. Liu, J. Gonzalez and S. S. Mao, Talanta, 2002, 57, 425–451 CrossRef CAS.
- C. A. Morrison, D. D. Lambert, R. J. S. Morrison, W. W. Ahlers and I. A. Nicholls, Chem. Geol., 1995, 119, 13–29 CrossRef CAS.
- N. J. G. Pearce, W. T. Perkins and R. Fuge, J. Anal. At. Spectrom., 1992, 7, 595–598 RSC.
- W. T. Perkins, R. Fuge and N. J. G. Pearce, J. Anal. At. Spectrom., 1991, 6, 445–449 RSC.
- M. Thompson, S. Chenery and L. Brett, J. Anal. At. Spectrom., 1989, 4, 11–16 RSC.
- J. M. Mermet and J. C. Ivaldi, J. Anal. At. Spectrom., 1993, 8, 795–801 RSC.
- M. Ducreux-Zappa and J. M. Mermet, Spectrochim. Acta, Part B, 1996, 51, 333–341 CrossRef.
- R. E. Russo, X. L. Mao, W. T. Chan, M. F. Bryant and W. F. Kinard, J. Anal. At. Spectrom., 1995, 10, 295–301 RSC.
- A. J. Amiel, G. M. Friedman and D. S. Miller, Sedimentology, 1973, 20, 47–64 CrossRef CAS.
- T. Mitsuguchi, T. Uchida, E. Matsumoto, P. J. Isdale and T. Kawana, Geochim. Cosmochim. Acta, 2001, 65, 2865–2874 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
