Speciation of gadolinium based MRI contrast agents in environmental water samples using hydrophilic interaction chromatography hyphenated with inductively coupled plasma mass spectrometry†
Chinthalapati Siva
Kesava Raju
a,
Antje
Cossmer
a,
Holger
Scharf
a,
Ulrich
Panne
ab and
Detlef
Lück
*a
aBAM Federal Institute for Materials Research and Testing, Richard-Willstaetter-Str. 11, 12489, Berlin, Germany. E-mail: detlef.lueck@bam.de; Fax: +49 30 8104 1117; Tel: +49 30 8104 1112
bHumboldt-Universitaet zu Berlin, Department of Chemistry, Brook-Taylor-Strasse 2, 12489, Berlin, Germany
First published on 23rd November 2009
Abstract
A highly sensitive method for the determination of gadolinium based MRI contrast agents in environmental water samples has been developed using hydrophilic interaction chromatography (HILIC) coupled with inductively coupled plasma mass spectrometry (ICP-MS). The contrast agents used in the present study are Gd-DTPA (Magnevist), Gd-DTPA-BMA (Omniscan), Gd-DOTA (Dotarem), Gd-BOPTA (Multihance) and Gd-BT-DO3A (Gadovist) which represent both classes of linear and macro cyclic and also ionic and non ionic contrast agents. A study of various parameters responsible for the efficient separation was performed and resulted in an optimum mobile phase composition of 20 mmol L−1 ammonium acetate in 60/40 ACN/water mixture at a flow rate of 0.1 mL min−1 in combination with a micro concentric type PFA nebuliser and ICP-MS detection of the 158Gd isotope. All contrast agents have shown excellent linearity between 0.1 μg L−1 and 100 μg L−1 (R2 > 0.99) for each MRI contrast agent with RSD values found to be less than 2% for triplicate measurements. The limit of quantification (LOQ) for all contrast agents were found to be less than 100 ng L−1 which allows the developed method to be employed in environmental trace analysis. Enrichment of water samples for the determination of contrast agents was performed by using a surface evaporation approach. The developed method has been applied successfully for the separation and determination of contrast agents in surface and waste water treatment plant samples. The MRI agent speciation results were compared with ICP-MS measurements for the total Gd concentrations.
Introduction
Magnetic resonance imaging (MRI) has become one of the most powerful techniques in early medical diagnostics and allows a detailed view of various parts of the body.1 Gadolinium containing MRI contrast agents are aqueous solutions that are injected into the body to improve the image quality and permit a more accurate picture to be observed.2,3 As gadolinium is highly toxic, it is reversibly chelated in a complex structure with other molecules in the contrast-agent solution. These complexes are very stable and thus exhibit much less toxicity compared to the free Gd3+ ion.4 Gd3+ has been shown to inhibit Ca2+ binding to mammalian cardiac sarcoplasmic reticulum. Acute toxicity of Gd3+ can cause ataxia, writhing, respiratory problems, sedation, hypotension and death by cardiovascular collapse.5In these contrast agents, the free Gd ions are commonly chelated by polyaminocarboxylic acid compounds. The chelating ligand significantly improves acute tolerance due to their binding sites and, furthermore, the Gd complexes are water-soluble.6 Two categories of Gadolinium contrast agents are currently in use, (a) “macrocyclic” chelates where Gd is “caged” in a pre-organized cavity of the polyamino-polycarboxylic ligand, and (b) “open-chain” (or “linear”) chelates. The structure and names of five commonly used contrast agents Gd-DTPA, Gd-DTPA-BMA, Gd-BOPTA, Gd-DOTA and Gd-BT-DO3A are given in Fig. 1. Gd-DTPA, Gd-DTPA-BMA and Gd-BOPTA belong to the open chain or linear chelates whereas Gd-DOTA and Gd-BT-DO3A belongs to the class of macrocyclic chelates. The second type of classification is based on ionic and non-ionic contrast agents. For ionic contrast agents (Gd-DTPA, Gd-BOPTA, and Gd-DOTA), meglumine is commonly used as a counter ion. Gd-DTPA-BMA and Gd-BT-DO3A belong to the class of non-ionic contrast agents.7
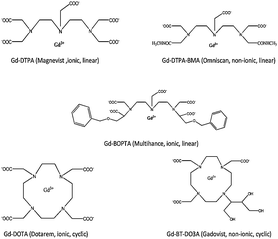 | ||
| Fig. 1 Chemical structures of five predominantly used MRI contrast agents. | ||
Nephrogenic systemic fibrosis (NSF), or nephrogenic fibrosing dermopathy, is a disease just recently described in patients who present firm, erythematous, and indurated plaques of the skin associated with subcutaneous edema. It may ultimately cause disabling contractures of several joints, thus making many patients wheelchair-dependent.8 NSF has been associated to prior administration of gadolinium chelates and more prone in patients affected with renal inefficiency.9–13 All these compounds are applied orally or intravenously at a dose of about 0.12 mmol Gd complex per kg body weight, which corresponds roughly to about 1.2 g of Gd per person (with an average body mass of 65 kg).14 It is estimated that 25–30% of all MRI investigations are performed with the help of contrast agents, resulting in about 20 million applications per year world wide.15 Clinical studies revealed that Gd complexes are excreted in non-metabolised form by the patient within few hours. After excretion, Gd complexes enter the hospital sewage system and, thereafter, the municipal sewage treatment plants and contaminate surface waters and subsequently river water. In the last few years, the total concentration of Gd increased in the environment resulting in a positive Gd-anomaly16 and in the vicinity of large cities, river and lake waters reveal distribution patterns with distinct positive Gd anomalies.17–20 Kümmerer and Helmers gave rough estimates of Gd consumption in German hospitals and private clinics of about 1160 kg per year.21 With natural concentrations of less than 10 pmolal Gd in surface water, it takes only 100 applications to double the Gd concentration of 1 km3 of surface water.22
Little is known about the behaviour of these compounds in environmental compartments. Mainly the total amount of Gd is determined in surface water or sewage sludge. Concentrations of gadolinium can be determined by using spectrometric techniques such as atomic absorption spectrometry,23 inductively coupled plasma atomic emission spectrometry,24 inductively coupled plasma mass spectrometry25 and X-ray fluorescence.26 However, these techniques are unable to distinguish the contrast agent and the various chemical species of gadolinium potentially present in the sample. To gather more detailed information about the Gd species, chromatographic techniques are required to separate the particular gadolinium compounds and to detect them individually. Compounds with hydrophilic properties like metal ion solutions are poorly retained by reversed phase interactions. In these cases, hydrophilic interaction liquid chromatography (HILIC) has recently emerged as a powerful alternative with improved performance.27,28 The zwitter ionic stationary phase has the advantage over common ion-exchangers of being charged independent of pH, which improves the possibilities to optimize the mobile phase composition with respect to the chemical properties of the analytes. For the speciation of hydrophilic metal compounds, HILIC chromatography is found to be an ideal choice. ICP-MS is the most sensitive detection method for the analysis of trace level metal concentrations. Hence, the combination HILIC-ICP-MS was chosen for the speciation and subsequent quantitative determination of Gd based contrast agents in the environment.
Experimental
Instrumentation
The chromatographic separation of contrast agents was performed using an Agilent 1100 series HPLC system comprising a G1379A micro vacuum degasser, a G1312A capillary pump, a G1313A autosampler, a G1316A column oven, and a G1314A variable wavelength detector (Agilent Technologies, Waldbronn, Germany), which was coupled to an Agilent 7500cx ICP-MS (Agilent, Waldbronn, Germany), employing a G3285 micro concentric PFA nebulizer and G3139 scott type spray chamber (Agilent, Germany) in combination with a water chiller (Van Der Heijden, Doerentrup, Germany). The detection was performed by a quadrupole mass spectrometer equipped with an octopole collision cell system. The software used for controlling the HPLC and ICP-MS data analysis was MSD ChemStation(Agilent). Quantification was performed in peak area mode and chromatograms were exported and plotted with Microsoft Excel software and Origin 8 software.HPLC conditions
The separation of Gd contrast agents was carried out using a zwitterionic ZIC-HILIC column (150 mm × 2.1 mm i.d., 3.5 μm particle size, 200 Å pore size, SeQuant GmbH, Marl, Germany). To prevent the contamination of the main column, a ZIC-HILIC pre-column (20 mm × 2.1 mm i.d., 5 μm particle size, 200 Å pore size, SeQuant GmbH) was used. The separation was performed with flow rate of 0.1 mL min−1. The optimised mobile phase used in this study consists of 20 mmol L−1 ammonium acetate in 60/40 acetonitrile/water (pH 5.8). The injection volume was optimised to 5 μL. The separation was performed in isocratic mode and the optimised separation time was 25 min. The optimised HPLC conditions are given in Table 1.| Instrument | Agilent HPLC 1100 |
| Column | Sequant ZIC-HILIC-Column (150 mm × 2.1 mm i.d.) |
| Mobile Phase | 20 mmol L−1 ammonium acetate in 60/40 ACN/water, pH 5.8 |
| Flow rate | 100 μL min−1 |
| Injection volume | 5 μL |
Hyphenation and ICP-MS conditions
The eluent from the column was transferred to ICP-MS via PEEK tubing with an inner diameter of 0.07 mm. All tubes and rubber sealings used are resistant to organic solvents. The sample introduction system consists of a pneumatic micro concentric PFA nebulizer with a scott-type spray chamber cooled by a peltier element to 2 °C. The considerable organic load of the mobile phase resulted in the deposition of elemental carbon on the quartz torch and on the walls of sample and skimmer cone during preliminary measurements. To prevent these effects, an optional gas flow (15%) in the form of Ar/O2 mixture (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) was added to the plasma gas. Direct measurement of total Gd was performed using auto sampler CETAC 501 (CETAC technologies, Omaha, USA) coupled with Agilent ICP-MS 7500cx. Both the direct measurements and hyphenated HPLC measurements with ICP-MS can be performed by varying the configuration of the instrumental settings. The optimised ICP-MS conditions are given in Table 2.
20) was added to the plasma gas. Direct measurement of total Gd was performed using auto sampler CETAC 501 (CETAC technologies, Omaha, USA) coupled with Agilent ICP-MS 7500cx. Both the direct measurements and hyphenated HPLC measurements with ICP-MS can be performed by varying the configuration of the instrumental settings. The optimised ICP-MS conditions are given in Table 2.
Reagents and standards
The gadolinium-based MRI contrast solutions were procured from their respective pharmaceutical companies. Gadovist (Gd-BT-DO3A, 1.0 mol L−1) and Magnevist (Gd-DTPA, 0.5 mol L−1) from Bayer Schering Pharma AG (Berlin, Germany), Omniscan (Gd-DTPA-BMA, 0.5 mol L−1) from GE Healthcare Buchler (Braunschweig, Germany), Dotarem (Gd-DOTA, 0.5 mol L−1) from Guerbet (Sulzbach, Germany), and Multihance (Gd-BOPTA, 0.5 mol L−1) from Nycomed GmbH (Konstanz, Germany).All chemicals were used in the highest quality available. Ammonium acetate was obtained from Merck KGaA (Darmstadt, Germany), HPLC grade acetonitrile was obtained from J. T. Baker (Deventer, Holland). Gadolinium standard stock solutions (1000 mg L−1 Gd for ICP-MS measurement, traceable to NIST SRM Gd2O3 in 2–3% HNO3, CertiPUR®) and internal standard solutions for ICP-MS measurement Ge (1000 mg L−1 Ge, traceable to NIST SRM (NH4)2GeF6 in H2O CertiPUR®) and Ho (1000 mg L−1 Ho traceable to NIST SRM Ho2O3 in 2–3% HNO3, CertiPUR®) were procured from Merck KGaA (Darmstadt, Germany). All solutions were prepared in 18 MΩ de-ionized water and filtered through a 0.22 μm Millipak 40 filter (Millipore, Molsheim, France). All dilute nitric acid solutions were prepared from freshly prepared sub boiling nitric acid.
Gadolinium contrast agent stock solutions were diluted with purified milliQ water to a concentration of 10 mmol L−1 and the resulting Gd mass concentration was calculated for each species. As the contrast agents are highly soluble in water, there is no need to add any further reagent. The calibration curves for contrast agents were performed with ten calibration solutions over a range of three decades (0.1 μg L−1 to 100 μg L−1). For the total Gd concentration measurements with ICP-MS, all standard solutions were prepared in 1% HNO3 solution by diluting the Gd standard stock solution. 5 μg L−1 Ge and Ho were added as internal standards for total ICP-MS measurements.
The HPLC mobile phase was prepared by dissolving the appropriate amount of ammonium acetate in water followed by addition of acetonitrile. The pH of the mobile phase was measured and the solution was filtrated prior to use as a mobile phase for chromatographic separation.
Sampling
Our sampling strategy was based on flow direction of the Berlin river streams from incoming to outgoing water. To confirm the contamination from manmade activities, samples were taken to represent the natural Berlin water flow of rivers Spree and Havel which flow through the city. The present study was performed on the incoming water to Berlin river Spree (Dämeritzsee) where it is mostly unpolluted, Lake Grosser Wannsee in the Berlin city and at Jungfernsee at Glinicker Brücke near Potsdam where river Havel is leaving Berlin.J. Künnemeyer et al.28 observed a decrease in contrast agents concentration when using glass bottles for sampling. Initial studies performed on our laboratory also confirms that there is slight decrease in the concentration because of the Gd adsorption on glass surfaces and it was found that the loss of analytes can be overcome if polypropylene or PFA type bottles are used. Hence, the sampling was performed in polypropylene bottles. The polypropylene bottles for the sampling were separated for HPLC-ICP-MS and total Gd ICP-MS direct measurements. The polypropylene bottles that were chosen to use for the total Gd determination were slightly acidified (0.2% HNO3 v/v) to adjust the pH to less than 2. All water samples were taken from a 0.5 m depth with polypropylene beaker connected to a special sampling apparatus. The sampling was done at different locations to have proper sampling statistics. Similar sampling was also performed in the waste water treatment plant. Special care was taken with clothing and gloves while handling the waste water samples. Sampling was performed in the waste water treatment plant near Wassmannsdorf in Berlin. The sampling points were the incoming water to the waste water treatment plant and also the outgoing water after the waste water treatment to have an idea about the purification and removal of these contrast agents. Due to the huge amount of treated water in this plant, the samples taken are not representative but are handled as random samples. Immediately after sampling, the solutions were filtered with a 0.45 μm membrane syringe filter and the filtrate solutions were stored in polypropylene bottles. The HPLC vials were also chosen to be made of polypropylene.
Enrichment of MRI agents in water samples
Though the present method is very sensitive for the determination of total Gd by direct ICP-MS, the quantitative speciation of contrast agents may not be successful as the concentration of individual contrast agents are below the quantification limit. In the developed method, weighed water samples were taken in pre-weighed PFA beakers and surface evaporation with IR light bulbs by gentle heating was performed without boiling. The samples were evaporated to approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 of the amount. The correction factor was calculated by the difference in the weight and applied during the calculation of the actual concentration. To ensure that there was no loss or destruction of Gd complexes, initial studies were performed with the mixture containing the contrast agents and evaporated to approximately 1
10 of the amount. The correction factor was calculated by the difference in the weight and applied during the calculation of the actual concentration. To ensure that there was no loss or destruction of Gd complexes, initial studies were performed with the mixture containing the contrast agents and evaporated to approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. No dissociation or other losses of the analytes were observed. Measurements for total Gd concentrations were also performed in a similar procedure.
20. No dissociation or other losses of the analytes were observed. Measurements for total Gd concentrations were also performed in a similar procedure.
Results and discussion
Method development
The separation of Gadolinium species were performed on a HILIC system coupled to an ICP-MS system. The optimised flow rate for this study was 0.1 mL min−1. The flow rate of the mobile phase plays a key role. A micro concentric PFA nebuliser allows a very favourable low flow rate. The higher the organic concentration in the mobile phase and its total flow, the higher will be the chance of clogging from organic carbon deposits on the torch and also on the cones of the ICP-MS system causing severe drift of the instrument. Though the flow of the mobile phase is very low (0.1 mL min−1), optional gas was added (15% Ar/O2 mixture, 80/20) to avoid the effect of carbon deposition formation if any. The maximum injection volume of 5 μL was limited through the HILIC column. The separation was studied with the different mobile phases initially and particularly using dimethyl formamide (DMF) as a mobile phase. The advantage of DMF is that solvent vapour is transported to the plasma with higher efficiency compared to ACN. DMF is a less volatile solvent which potentially reduces carbon depositions and improve sensitivity.29 The DMF based separation method developed was able to separate the five contrast agents, but without baseline separation of peaks which makes quantification difficult. Hence, in the present study a mixture of ACN and water in ammonium acetate buffer was chosen as the mobile phase. The ACN concentration of the mobile phase and buffer concentration were optimised for an effective separation followed by quantitative determination.Mobile phase and buffer variation
Hydrophilic interaction chromatography (HILIC) is based on analyte retention through partitioning of the analyte between a water-enriched layer of stagnant eluent on a hydrophilic stationary phase and a relatively hydrophobic bulk eluent, with the main components usually being 5–40% water in ACN. The composition of the mobile phase plays a vital role in the separation of hydrophilic compounds. Also, another important factor for HILIC separations is the buffer concentration. The use of buffered eluents in HILIC reduces the electrostatic interactions between charged analytes and deprotonated silanol groups of the stationary phase. Ammonium salts as formate or acetate are mostly recommended due to their high solubility in eluents with high ACN concentrations and because their buffering range is suitable for most HILIC applications. The elution behaviour with different buffer solutions was preliminary performed and ammonium acetate was found to be the ideal choice for the separation.The experiments were performed on the variation of ACN in the mobile phase. The acetonitrile percentage in the mobile phase varied from 30% to 90% keeping the ammonium acetate percentage constant in all mobile phase solutions. Separation was performed with the mixture containing all five contrast agents. The chromatographic separation is shown in Figure S1 in the ESI.† The acetonitrile concentration was found to be the major influence in the separation process. With an increase of the acetonitrile concentration in the mobile phase, a strong retention of the contrast agents was observed. Only Gd-BOPTA was separated even after 40 min. At lower concentration of ACN (<40%) a fast separation is possible but the ionic contrast agents such as Gd-BOPTA and Gd-DTPA and non ionic Gd-BT-DO3A and Gd-DTPA-BMA were eluted together. The exact mechanism for the retention of contrast agents is not well understood. Both the zwitter ionic stationary phase and the mobile phase play an important role in the retention. The mobile phase composition was optimised to 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 ACN/H2O where all contrast agents were separated nearly to the base line. The buffer concentration varied (5 mmol L−1 to 40 mmol L−1) and the resulting chromatograms are given as Figure S2 (ESI).† It was observed that with an increase in the concentration of ammonium acetate, the retention of contrast agents became more prominent and at lower concentrations, some of the contrast agents were eluting together. Ammonium acetate was found to be major influence in the separation. Electrostatic repulsion of acidic or negatively charged species can lead to complete expulsion of charged species from the column.30 The early elution of ionic contrast agents compared to the non-ionic contrast agents may be because of the influence of buffer concentration. The elution order was not changed with the buffer concentration. However, with the increase in the buffer concentration, the separation between Gd-BOPTA, Gd-DTPA and Gd-DOTA was more prominent. The separation of non-ionic contrast agents, Gd-BT-DO3A and Gd-DTPA-BMA was shown to be independent of the buffer variation. Hence, the optimised mobile phase composition for the speciation of contrast agents was maintained at 20 mmol L−1 ammonium acetate in 60/40 ACN/water. The time of analysis for a single run was then 25 min.
40 ACN/H2O where all contrast agents were separated nearly to the base line. The buffer concentration varied (5 mmol L−1 to 40 mmol L−1) and the resulting chromatograms are given as Figure S2 (ESI).† It was observed that with an increase in the concentration of ammonium acetate, the retention of contrast agents became more prominent and at lower concentrations, some of the contrast agents were eluting together. Ammonium acetate was found to be major influence in the separation. Electrostatic repulsion of acidic or negatively charged species can lead to complete expulsion of charged species from the column.30 The early elution of ionic contrast agents compared to the non-ionic contrast agents may be because of the influence of buffer concentration. The elution order was not changed with the buffer concentration. However, with the increase in the buffer concentration, the separation between Gd-BOPTA, Gd-DTPA and Gd-DOTA was more prominent. The separation of non-ionic contrast agents, Gd-BT-DO3A and Gd-DTPA-BMA was shown to be independent of the buffer variation. Hence, the optimised mobile phase composition for the speciation of contrast agents was maintained at 20 mmol L−1 ammonium acetate in 60/40 ACN/water. The time of analysis for a single run was then 25 min.
Figures of merit
To determine the figures of merit, calibration curves for each contrast agent were performed by analysing the concentration in the range of 0 to 100 μg L−1 Gd (0 to 0.63 μmol L−1 Gd). The calibration curves for the gadolinium(III) complexes were linear over the wide concentration range of 0.1 to 100 μg L−1 Gd (0.63 nmol L−1 to 0.63 μmol L−1Gd) with ten calibration points. The correlation coefficient for each contrast agent was found to be higher than 0.99.Each analysis was performed in triplicate and the average of three measurements was taken for the calculation of linearity. The relative standard deviations (RSD) of peak areas as a measure for repeatability were found to be less than 2%. The mean RSD value for 1 μg L−1 of each contrast agents is given in Table 3.
| Gd complex | LOD in ng L−1 Gd (nmol L−1 Gd) | LOQ in ng L−1 Gd (nmol L−1 Gd) | Repeatability RSD in % | Linearity (R2) |
|---|---|---|---|---|
| Gd-BOPTA | 21 (0.13) | 71 (0.44) | 1.2 | 0.998 |
| Gd-DTPA | 28 (0.17) | 92 (0.57) | 0.9 | 0.991 |
| Gd-DOTA | 17 (0.11) | 57 (0.35) | 1.7 | 0.995 |
| Gd-DTPA-BMA | 18 (0.12) | 60 (0.38) | 1.0 | 0.993 |
| Gd-BT-DO3A | 24 (0.15) | 81 (0.50) | 1.5 | 0.990 |
The limits of detection (LOD) were determined by means of a signal-to-noise ratio of three (S/N = 3) with standard solutions without enrichment. The limits of quantification (LOQ) were determined for a signal-to-noise ratio of ten (S/N = 10). Both LOD and LOQ were calculated on the basis of 158Gd abundance. The LOD was found to be in the range at 22 ± 5 ng L−1 Gd for each contrast agent and the LOQ was found to be 72 ± 15 ng L−1 Gd using the developed method. Single LOD, LOQ and regression coefficient values are given in Table 3. Differences in numbers are based on integration variations of low concentration peaks. The LOD values obtained in the present method have been found to be more sensitive compared to the other literature reported values. A comparison table is given in Table 4 in which the more sensitive Gd complex is taken as reference. The direct ICP-MS measurement for the present study was found to be more sensitive with LOD and LOQ values were found to be 6 ng/L and 20 ng/L respectively. Summarizing, this approach proved to be more sensitive than any other reported method due to the excellent separation offered by HILIC and the efficient determination by ICP-MS.
| Gd complex | Method | LOD in ng L−1 Gd | Application | Reference |
|---|---|---|---|---|
| Gd-DOTA | HILIC-ICP-MS | 17 | Surface water samples | Present work |
| Gd-DOTA | HPLC-ICP-OES | 7850 | Blood samples | 31 |
| Gd-DOTA | HILIC-ESI-MS | 15700 | Blood samples | 27 |
| Gd-DOTA | HILIC-ICP-MS | 157 | Waste water samples | 28 |
| Gd-DTPA | ICP-MS | 200 | River water samples | 32 |
| Gd-DTPA-BMA | HPLC-UV (PCR with Arsenazo-III) | 172000 | Serum and faeces | 33 |
The separation of contrast agents at trace concentrations (0.5 μg L−1 Gd) is given in Fig. 2. These features enable this method to be employed for environmental water samples which require a highly sensitive separation and quantification method.
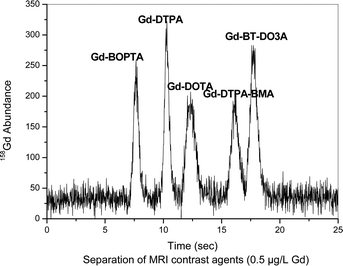 | ||
| Fig. 2 Separation of contrast agents at a concentration of 0.5 μg L−1 Gd each. Column: Sequant ZIC-HILIC-Column (150 mm × 2.1 mm i.d.), mobile phase: 20 mmol/lL ammonium acetate in 60/40% ACN/H2O, flow rate: 0.1 mL min−1, detection: as 158Gd with ICP-MS, sample: contrast agent mixture with each 0.5 μg L−1 Gd, sample injected: 5 μL. | ||
Applications to surface water samples and samples collected at waste water treatment plants
To test the developed method for the determination of contrast agents in the environment, it was applied for the separation of contrast agents in surface water samples collected at different locations in Berlin. The surface water system of Berlin, Germany, is located in the outwash plain of the last glacial period. Berlin is a densely populated area with 3.5 million inhabitants over 891 km2. Glacial erosion and drainage during the ice melt created the rivers Dahme, Spree and Havel, and their lake-like widenings form Lake Tegel, Lake Wannsee and Lake Müggelsee. The low slope of the relief (≈ 0.1%) induces very low flow rates for the river waters (about 1 km per day).34 Initial experiments with ICP-MS for total Gd measurement have shown that natural water concentrations of total Gd are lower than 100 ng L−1. Speciation measurements of each contrast agent at that low concentration is an extremely difficult task and may not be possible due to the higher LOQ. Therefore, water samples were concentrated by the surface evaporation method to approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 of the total volume. The individual enrichment factor for samples was calculated based on gravimetric measurements. The separation of contrast agents was performed from the water samples collected at incoming water to Berlin river Spree (collected at Dämeritzsee), in Berlin Lake Wannsee, River Havel and outgoing water leaving Berlin collected at Jungfernsee at Glinicker Brücke near Potsdam. The corresponding HILIC-ICP-MS spectra for the contrast agent separation is displayed in Fig. 3.
10 of the total volume. The individual enrichment factor for samples was calculated based on gravimetric measurements. The separation of contrast agents was performed from the water samples collected at incoming water to Berlin river Spree (collected at Dämeritzsee), in Berlin Lake Wannsee, River Havel and outgoing water leaving Berlin collected at Jungfernsee at Glinicker Brücke near Potsdam. The corresponding HILIC-ICP-MS spectra for the contrast agent separation is displayed in Fig. 3.
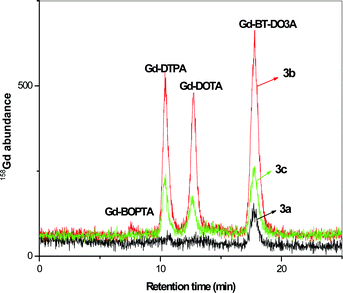 | ||
| Fig. 3 Separation of contrast agents from environmental samples after 1 to 10 enrichment by surface evaporation 3a) incoming water to Berlin from river Spree collected at Dämeritzsee, 3b) water sample collected at Wannsee, Berlin, 3c) outgoing water from Berlin collected at Jungfernsee, Glinicker Brücke near Potsdam. Concentrations are given in Table 5. Column: Sequant ZIC-HILIC-Column (150 mm × 2.1 mm i.d.), mobile phase: 20 mmol L−1 ammonium acetate in 60/40% ACN/H2O, flow rate: 0.1 mL min−1, detection: as 158Gd with ICP-MS, sample injected: 5 μL. | ||
It was observed that the cyclic Gd-BT-DO3A was present in all the samples. In Dämeritzsee, which is the incoming water to Berlin, only trace amounts of Gd-BT-DO3A were found. In both samples collected in Grosser Wannsee and at Jungfernsee at Glinicker Brücke, Gd-DOTA and Gd-DTPA were present along with Gd-BT-DO3A. The absence of linear chain complexes, such as Gd-DTPA-BMA and Gd-BOPTA (though present as a trace in the Wannsee sample), may be due to the fact that today many hospitals avoid these contrast agents because of the adverse effects observed in patients at the end stage of renal disease resulting in nephrogenic systemic fibrosis28 and, hence, linear contrast agents are less frequently applied. The appearance of Gd-DTPA in the samples at Wannsee and Jungfernsee may be due to the fact that it is a widely used contrast agent and still remains undissociated in the water samples.
The presence of cyclic contrast agents in the samples is expected as they are more stable complexes compared to linear contrast agents and less prone to release the Gd.35 The concentrations of contrast agents in the water samples collected at Jungfernsee are comparatively lower than the concentrations in Wannsee. This may be due to the dilution of water that can be possible during the course of flow. However, when compared to the incoming water to Berlin, the concentration of Gd increased approximately 15 times clearly indicating contamination due to Gd contrast agents mainly originating from hospital effluents into the environment. The detailed concentration of each contrast agents and the compared Gd total value by ICP-MS measurement are given in Table 5.
| Sample | Gd-BOPTA (ng L−1 Gd) | Gd-DTPA (ng L−1 Gd) | Gd-DOTA (ng L−1 Gd) | Gd-DTPA-BMA (ng L−1 Gd) | Gd-BT-DO3A (ng L−1 Gd) | ∑ Gd complex (ng L−1 Gd) | ∑ Gd direct (ng L−1 Gd) |
|---|---|---|---|---|---|---|---|
| Dämeritzsee | nd | nd | nd | nd | 12 | 12 | 15 |
| Wannsee | 12 | 106 | 110 | nd | 184 | 412 | 436 |
| Jungfernsee | nd | 54 | 50 | nd | 89 | 194 | 215 |
| WWTP Wassmannsdorf, incoming water | nd | 29 | 34 | nd | 36 | 99 | 122 |
| WWTP Wassmannsdorf, outgoing water | nd | 27 | 36 | nd | 34 | 97 | 118 |
Samples collected from a waste water treatment plant displayed no significant removal of the Gd agents. Similar concentrations for samples from incoming and outgoing water were observed. The reason may be that these contrast agents are very stable and strongly bind Gd and are highly water miscible, thus remaining undissociated during the various treatment steps. The speciation of contrast agents from the waste water treatment water samples is revealed in Fig. 4.
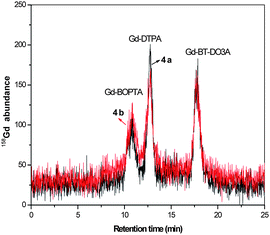 | ||
| Fig. 4 Separation of contrast agents from waste water treatment samples. 4a) Incoming water to waste water treatment plant, Wassmannsdorf, Berlin, 4b) outgoing water from waste water treatment plant, Wassmannsdorf, Berlin. Concentrations given in Table 5. Column: Sequant ZIC-HILIC-Column (150 mm × 2.1 mm i.d.), mobile phase: 20 mmol L−1 ammonium acetate in 60/40% ACN/H2O, flow rate: 0.1 mL min−1, detection: as 158Gd with ICP-MS, sample injected: 5 μL. | ||
The main contrast agents present in the waste water treatment samples were found to be Gd-DTPA, Gd-DOTA, and Gd-BT-DO3A, similar to the samples collected at Wannsee and Jungfernsee. There is an increase in the concentration of total Gd measured directly with ICP-MS when compared to the sum of the gadolinium contrast agents when measured with HILIC-ICP-MS. This may be due to the ionic Gd available in the water samples.
The free Gd shows very high retention on the HILIC stationary phase and elution is not feasible. If there is any trans-metallation reaction experienced by the contrast agents, then there is a chance that free Gd can be liberated from the complex resulting in the high concentration of Gd in the environment.36 The results of HILIC-ICP-MS compared to ICP-MS by direct measurement for the waste water samples are given in Table 5.
Conclusions
A HILIC-ICP-MS method for the baseline separation and quantification of five contrast agents namely, Gd-DTPA, Gd-DTPA-BMA, Gd-BOPTA, Gd-DOTA and Gd-BT-DO3A that are predominantly used in MRI applications was developed. The developed method offers excellent sensitivity down to the ppt range (LOQ values found to be < 100 ng L−1) allowing the method to be used in eco-toxicological analysis. Chromatographic parameters responsible for sufficient separation and calibration of MRI complexes were optimised. The advantage of this method is that is very specific and can be applied with minimal sample pre-treatment. The method was successfully applied to environmental water samples and also water samples collected at a waste water treatment plant. The results indicate that the Gadolinium concentration is increasing in the environment due to hospital waste disposal and contrast agents escaping the waste water treatment process steps.Acknowledgements
We are thankful to the Alexander von Humboldt foundation for sponsoring the fellowship of Dr Chinthalapati Siva Kesava Raju to carry out this work. We are also thankful to WWTP, Wassmannsdorf to collect waste water treatment plant samples. We thank Prof. Dr Uwe Karst’s group, University of Münster and Charité Berlin-Steglitz hospital for providing samples of contrast agents.Notes and References
- R. B. Lauffer, Chem. Rev., 1987, 87, 901–927 CrossRef CAS.
- E. Moutiez, P. Prognon, P. Bourrinet, S. Zehaf, A. Dencausse and G. Mahuzier, Analyst, 1997, 122, 1347–1352 RSC.
- M. F. Bellin, M. Vasile and S. Morel-Precetti, Eur. Radiol., 2003, 13, 2688–2698 CrossRef CAS.
- A. Bianchi, L. Calabi, F. Corana, S. Fontana, P. Losi, A. Maiocchi, L. Paleari and B. Valtancoli, Coord. Chem. Rev., 2000, 204, 309–3935 CrossRef CAS.
- S. M. Rocklage, D. Worah and S. H. Kim, Magn. Reson. Med., 1991, 22(2), 216–221 CrossRef CAS.
- H. Ersoy and F. J. Rybicki, J. Magn. Reson. Imaging, 2007, 26, 1190–1197 CrossRef.
- P. Caravan, J. J. Ellison, T. J. McMurry and R. B. Lauffer, Chem. Rev., 1999, 99, 2293–2352 CrossRef CAS.
- S. E. Cowper, Curr. Opin. Rheumatol., 2003, 15, 785–790 CrossRef.
- J. M. Idee, M. Port, C. Medina, E. Lancelot, E. Fayoux, S. Ballet and C. Corot, Toxicology, 2008, 248, 77–88 CrossRef CAS.
- T. Grobner, Nephrol., Dial., Transplant., 2006, 21, 1104–1108 CAS.
- P. Marckmann, L. Skov, K. Rossen, A. Dupont, M. B. Damholt, J. G. Heaf and H. S. Thomsen, J. Am. Soc. Nephrol., 2006, 17, 2359–2362 CrossRef.
- S. E. Cowper, Adv. Dermatol., 2007, 23, 131–154 Search PubMed.
- S. E. Cowper, H. S. Robin, S. M. Steinberg, L. D. Su, S. Gupta and P. E. LeBoit, Lancet, 2000, 356, 1000–1001 CrossRef CAS.
- P. Möller, G. Morteani and P. Dulski, Acta Hydrochim. Hydrobiol., 2003, 31, 225–239 CrossRef.
- J. M. Idee, M. Port, I. Raynal, M. Schaefer, S. Le Greneur and C. Corot, Fundam. Clin. Pharmacol., 2006, 20, 563–576 CrossRef CAS.
- M. Bau and P. Dulski, Earth Planet. Sci. Lett., 1996, 143, 245–255 CrossRef CAS.
- Y. Nozika, D. Lerche, D. S. Alibo and M. Tsutsumi, Geochim. Cosmochim. Acta, 2000, 64, 3975–3982 CrossRef.
- P. Möller, T. Paces, P. Dulski and G. Morteani, Environ. Sci. Technol., 2002, 36, 2387–2394 CrossRef CAS.
- Y. Nozika, D. Lerche, D. S. Alibo and A. Sinidvongs, Geochim. Cosmochim. Acta, 2000, 64, 3983–3994 CrossRef.
- M. Bau, A. Knappe and P. Dulski, Chem. Erde, 2006, 66, 143–152 Search PubMed.
- K. Kümmerer and E. Helmers, Environ. Sci. Technol., 2000, 34, 573–577 CrossRef.
- P. Möller, Dulski, M. Bau, A. Knappe, A. Pekdeger and C. Sommer-von Jarmersted, J. Geochem. Explor., 2000, 69–70, 409–414 CrossRef CAS.
- L. Liang, P. C. D'Haese, L. V. Lamberts, F. L. Van de Vyver and M. E. De Broe, Anal. Chem., 1991, 63, 423–427 CrossRef CAS.
- J. G. Crock and F. E. Lichte, Anal. Chem., 1982, 54, 1329–1332 CAS.
- K. Hennebrüder, R. Wennrich, J. Mattusch, H. J. Stärk and W. Engewald, Talanta, 2004, 63, 309–316 CrossRef CAS.
- M. Lal, R. K. Choudhury and R. M. Agrawal, X-Ray Spectrom., 1987, 16, 23–26 CAS.
- J. Künnemeyer, L. Terborg, S. Nowak, A. Scheffer, L. Telgmann, F. Tokmak, A. Günsel, G. Wiesmüller, S. Reichelt and U. Karst, Anal. Chem., 2008, 80, 8163–8170 CrossRef CAS.
- J. Künnemeyer, L. Terborg, B. Meermann, C. Brauckmann, I. Möller, A. Scheffer and U. Karst, Environ. Sci. Technol., 2009, 43, 2884–2890 CrossRef.
- Y. Nygren, P. Hemström, C. Astot, P. Naredi and E. Björn, J. Anal. At. Spectrom., 2008, 23, 948–954 RSC.
- P. Hemstroem and K. Irgum, J. Sep. Sci., 2006, 29, 1784–1821 CrossRef.
- C. L. Kahakachchi and D. A. Moore, J. Anal. At. Spectrom., 2009, 24, 1389 RSC.
- K. Hennebrüder, R. Wennrich, J. Mattusch, H. J. Stärk and W. Engewald, Talanta, 2004, 63, 309–316 CrossRef CAS.
- P. T. Normann, P. Joffe, I. Martinsen and H. S. Thomsen, J. Pharm. Biomed. Anal., 2000, 22, 939–947 CrossRef CAS.
- A. Knappe, P. Möller, P. Dulski and A. Pekdeger, Chem. Erde, 2005, 65, 167–189 Search PubMed.
- R. Agarwal, S. M. Brunelli, K. Williams, M. D. Mitchell, H. I. Feldman and C. A. Umscheid, Nephrol., Dial., Transplant., 2009, 24, 856–863 CAS.
- N. R. Puttagunta, W. A. Gibby and G. T. Smith, Invest. Radiol., 1996, 31, 739–742 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Figures S1 and S2. See DOI: 10.1039/b919959d |
| This journal is © The Royal Society of Chemistry 2010 |
