Intracellular imaging of HeLa cells by non-functionalized NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) Er3+, Yb3+ upconverting nanoparticles†
Er3+, Yb3+ upconverting nanoparticles†
Fiorenzo
Vetrone
a,
Rafik
Naccache
a,
Angeles
Juarranz de la Fuente
b,
Francisco
Sanz-Rodríguez
b,
Alfonso
Blazquez-Castro
b,
Emma Martin
Rodriguez
c,
Daniel
Jaque
c,
José García
Solé
*c and
John A.
Capobianco
*a
aDepartment of Chemistry and Biochemistry, Concordia University, 7141 Sherbrooke St. W., Montreal, QC H4B 1R6, Canada. E-mail: capo@vax2.concordia.ca
bDepartamento de Biología, Facultad de Ciencias, Universidad Autónoma de Madrid, 28049 Madrid, Spain
cGIEL, Departamento de Física de Materiales, C-IV, Universidad Autónoma de Madrid, C/Francisco Tomás y Valiente 7, 28049 Madrid, Spain. E-mail: jose.garcia_sole@uam.es
First published on 17th November 2009
Abstract
We report on the efficient incorporation of non-functionalized NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Yb3+nanoparticles inside HeLa live cancer cells by direct endocytosis. The efficient two-photon excited near-infrared-to-visible upconversion fluorescence of these nanoparticles is then used to obtain high-contrast intracellularfluorescence images of single cells. These images reveal a redistribution of the nanoparticles inside the cell as the incubation time increases. Thus, non-functionalized NaYF4
Er3+, Yb3+nanoparticles inside HeLa live cancer cells by direct endocytosis. The efficient two-photon excited near-infrared-to-visible upconversion fluorescence of these nanoparticles is then used to obtain high-contrast intracellularfluorescence images of single cells. These images reveal a redistribution of the nanoparticles inside the cell as the incubation time increases. Thus, non-functionalized NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Yb3+nanoparticles emerge as very promising fluorescence probes for real-time imaging of cellular dynamics.
Er3+, Yb3+nanoparticles emerge as very promising fluorescence probes for real-time imaging of cellular dynamics.
Fluorescence imaging is a powerful non-invasive tool playing an integral role in biomedicine due to its high sensitivity and resolution.1 Typical fluorescence imaging techniques rely on fluorophore labels that are excited with UV light. In vivo imaging using UV excitation has well-known drawbacks including low tissue penetration depth, autofluorescence from other fluorophores in the sample medium, as well as the possibility of damaging the specimen under study.2 While the sensitivity and resolution of fluorescence imaging reported to date is acceptable, it could be further improved if the excitation of the fluorescence probes made use of multi-photon processes when exciting with near-infrared (NIR) light.3–5 The use of NIR radiation within the “optical transmission window” of biological tissues (700–1000 nm) increases the optical contrast since it simultaneously leads to greater light penetration depths, to a minimal autofluorescence contribution and to reduced scattering.2,6,7 Moreover, NIR light does not exhibit the same negative effect on cell function and organelles that UV radiation does.8,9 In addition to all these advantages, the use of femtosecond NIR pulses minimizes thermal loading and thus, tissue damage. Minimizing the tissue damage affords the possibility of undertaking imaging experiments requiring long measurement times such as those related to bio-dynamics (of special relevance in fast developing cells, for example cancer cells). Therefore, fluorescence probes showing efficient and stable multi-photon optical conversion in the visible following femtosecond NIR excitation are required for time-resolved intracellular imaging.
Over the last few years, the rapid progression of nanotechnology has propelled the development of novel light-emitting nanoparticles (NPs) capable of being excited with NIR light for use in multi-photon imaging. Their reduced size (few tens of nanometres) allows for more facile cell penetration, making intracellular imaging as well as photodynamic therapy a real possibility.10–13Quantum dots (QDs)14,15 and metallic nanoparticles,16 in particular gold nanorods (GNRs),17 of different sizes have recently been developed and used as two-photon fluorescence biolabels, leading to high-contrast images of live cells.18 In both cases the two-photon emission spectra obtained following NIR excitation are strongly dependent on their shape and local environment. In addition, there is concern about their toxicity owing to the presence of metals in their composition. Thus, there still exists a need to develop new materials that can overcome some of the drawbacks associated with the current crop of fluorophores while maintaining a low toxicity profile.
Lanthanide (Ln3+) doped nanoparticles, in particular NaYF4NPs co-doped with ytterbium (Yb3+) and erbium (Er3+) or thulium (Tm3+) ions, have recently emerged as a serious alternative to both QDs and GNRs for biomedical imaging.19–24NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+, Yb3+nanoparticles (Ln = Er or Tm) can be prepared via various techniques and are dispersible in several solvents (including water) to form colloidally stable solutions.25–29 They present a high long-term fluorescence stability, low toxicity as well as high fluorescence quantum yield. Furthermore, they can be excited via multi-photon absorption of NIR photons and since the Ln3+ dopant ions posses “real” excited intermediate states, relatively low excitation power levels can be used, thus facilitating the development of suitable instrumentation for low-cost detection. This multi-photon process, typically referred to as upconversion, converts the NIR excitation light to higher energies such as visible or UV30,31 (for a description of upconversion see the ESI† ). The two-photon excited fluorescence (upconversion) spectrum obtained from NaYF4
Ln3+, Yb3+nanoparticles (Ln = Er or Tm) can be prepared via various techniques and are dispersible in several solvents (including water) to form colloidally stable solutions.25–29 They present a high long-term fluorescence stability, low toxicity as well as high fluorescence quantum yield. Furthermore, they can be excited via multi-photon absorption of NIR photons and since the Ln3+ dopant ions posses “real” excited intermediate states, relatively low excitation power levels can be used, thus facilitating the development of suitable instrumentation for low-cost detection. This multi-photon process, typically referred to as upconversion, converts the NIR excitation light to higher energies such as visible or UV30,31 (for a description of upconversion see the ESI† ). The two-photon excited fluorescence (upconversion) spectrum obtained from NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Yb3+NPs under NIR femtosecond excitation (100 fs, 920 nm, excitation density = 0.1 GW m−2) (shown in Fig. 1 together with those of QDs14 and GNRs18) is characterized by two well-defined structured emission bands in the green and red spectral ranges. These bands are respectively assigned to the (2H11/2, 4S3/2) → 4I15/2 and 4F9/2 → 4I15/2 transitions of the Er3+ ion, which occur as a result of an efficient Yb3+ → Er3+ energy transfer.30 The spectral positions of these bands have been found to be independent of the NP size, shape and environment, this being an advantage over bands of both QDs and GNRs obtained after two-photon excitation, which are highly sensitive to those parameters.32,33 In addition, the NaYF4
Er3+, Yb3+NPs under NIR femtosecond excitation (100 fs, 920 nm, excitation density = 0.1 GW m−2) (shown in Fig. 1 together with those of QDs14 and GNRs18) is characterized by two well-defined structured emission bands in the green and red spectral ranges. These bands are respectively assigned to the (2H11/2, 4S3/2) → 4I15/2 and 4F9/2 → 4I15/2 transitions of the Er3+ ion, which occur as a result of an efficient Yb3+ → Er3+ energy transfer.30 The spectral positions of these bands have been found to be independent of the NP size, shape and environment, this being an advantage over bands of both QDs and GNRs obtained after two-photon excitation, which are highly sensitive to those parameters.32,33 In addition, the NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Yb3+NPs fluorescence bands show a clear structure, as opposed to the fluorescence bands generated by QDs and GNRs, which lack any fine structure. This structure is caused by the simultaneous emission from different close electronic energy levels of the Er3+ ions, whose population is determined by the environment temperature. As a result, NaYF4
Er3+, Yb3+NPs fluorescence bands show a clear structure, as opposed to the fluorescence bands generated by QDs and GNRs, which lack any fine structure. This structure is caused by the simultaneous emission from different close electronic energy levels of the Er3+ ions, whose population is determined by the environment temperature. As a result, NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Yb3+NPs could in the future, offer the additional possibility of being used as in vivo thermal nano-probes. Finally, the emission bands of the NaYF4
Er3+, Yb3+NPs could in the future, offer the additional possibility of being used as in vivo thermal nano-probes. Finally, the emission bands of the NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Yb3+NPs are narrower than those of QDs and GNRs (see Fig. 1), allowing for the achievement of high-spectral-contrast images by better discrimination of cell autofluorescence (if any). These outstanding features make NaYF4
Er3+, Yb3+NPs are narrower than those of QDs and GNRs (see Fig. 1), allowing for the achievement of high-spectral-contrast images by better discrimination of cell autofluorescence (if any). These outstanding features make NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Yb3+NPs excellent candidates for the next generation of fluorescent nano-probes for in vivo bio-imaging. Indeed, functionalized NaYF4
Er3+, Yb3+NPs excellent candidates for the next generation of fluorescent nano-probes for in vivo bio-imaging. Indeed, functionalized NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Yb3+NPs have been very recently linked to antibodies (NP conjugates) for specific labeling of cancer cells.22,23,34 These functionalized NPs attached specifically to the membranes of HeLa cancer cells by means of antibody–antigen affinity, thus allowing for detection and surface imaging of these cells. Nevertheless, for intracellular imaging the luminescent NPs should be incorporated on the inside of the cancer cell rather that remaining attached on their surface. For this purpose, specific functionalization of the surface of NPs would not be required. Despite the significant interest, to our knowledge no attempt to obtain intracellular images of live cancer cells by the two-photon emission of NaYF4
Er3+, Yb3+NPs have been very recently linked to antibodies (NP conjugates) for specific labeling of cancer cells.22,23,34 These functionalized NPs attached specifically to the membranes of HeLa cancer cells by means of antibody–antigen affinity, thus allowing for detection and surface imaging of these cells. Nevertheless, for intracellular imaging the luminescent NPs should be incorporated on the inside of the cancer cell rather that remaining attached on their surface. For this purpose, specific functionalization of the surface of NPs would not be required. Despite the significant interest, to our knowledge no attempt to obtain intracellular images of live cancer cells by the two-photon emission of NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Yb3+NPs has been reported to date.
Er3+, Yb3+NPs has been reported to date.
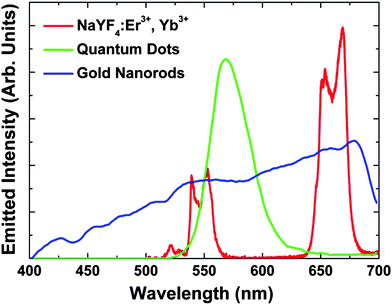 | ||
Fig. 1
Two-photon visible emission spectrum generated by the NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Yb3+NPs (1 wt% in water) used in this work as obtained after excitation with femtosecond NIR pulses (920 nm, 100 fs) showing the green 2H11/2, 4S3/2 → 4I15/2 emission (500 to 575 nm) and the red 4F9/2 → 4I15/2 emission (625 to 700 nm). The two-photon emission spectra obtained from GNRs (10–15 nm in length)18 and QDs (3.4 nm)14 under fs excitation are also shown for comparison. Er3+, Yb3+NPs (1 wt% in water) used in this work as obtained after excitation with femtosecond NIR pulses (920 nm, 100 fs) showing the green 2H11/2, 4S3/2 → 4I15/2 emission (500 to 575 nm) and the red 4F9/2 → 4I15/2 emission (625 to 700 nm). The two-photon emission spectra obtained from GNRs (10–15 nm in length)18 and QDs (3.4 nm)14 under fs excitation are also shown for comparison. | ||
In this work water dispersible (non-functionalized) NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Yb3+ upconverting NPs have been synthesized and then efficiently incorporated inside HeLa cancer cells by a simple incubation treatment and subsequent direct endocytosis. This allowed us to obtain high-contrast two-photon fluorescence intracellular images of individual HeLa cells with total absence of autofluorescence. In order to explore the ability of NaYF4
Er3+, Yb3+ upconverting NPs have been synthesized and then efficiently incorporated inside HeLa cancer cells by a simple incubation treatment and subsequent direct endocytosis. This allowed us to obtain high-contrast two-photon fluorescence intracellular images of individual HeLa cells with total absence of autofluorescence. In order to explore the ability of NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Yb3+NPs for the study of intracellular dynamics, single-cell images were obtained after different incubation times and then compared.
Er3+, Yb3+NPs for the study of intracellular dynamics, single-cell images were obtained after different incubation times and then compared.
The NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Yb3+NPs with dopant concentrations of 2% Er3+ and 18% Yb3+ used in this work were fabricated via a solvothermal synthetic route.28 In a typical experiment, 3.6 mmol of NaCl, 1.44 mmol of YCl3·6H2O, 0.036 mmol of ErCl3·6H2O, and 0.324 mmol of YbCl3·6H2O are dissolved in a 27 mL solution of ethylene glycol containing 0.45 g of branched polyethyleneimine (Mw ∼25,000) and stirred for approximately 60 min. Subsequently, a solution of 17 mL ethylene glycol with 7.2 mmol NH4F is added to the initial solution containing the chlorides and stirred for another 30 min. The resulting clear solution is then heated in a 250 mL Teflon lined autoclave (Berghof/America) with stirring for 24 h at 200 °C. The resulting nanoparticles may be isolated viacentrifugation using a mixture of distilled water and ethanol. TEM analysis showed the nanoparticles to be nearly spherical in shape with an approximate diameter of 18 nm (see Fig. S2 of the ESI† ). The PEI-capped nanoparticles crystallize in the cubic phase (α-NaYF4 phase) as was confirmed by XRD analysis.
Er3+, Yb3+NPs with dopant concentrations of 2% Er3+ and 18% Yb3+ used in this work were fabricated via a solvothermal synthetic route.28 In a typical experiment, 3.6 mmol of NaCl, 1.44 mmol of YCl3·6H2O, 0.036 mmol of ErCl3·6H2O, and 0.324 mmol of YbCl3·6H2O are dissolved in a 27 mL solution of ethylene glycol containing 0.45 g of branched polyethyleneimine (Mw ∼25,000) and stirred for approximately 60 min. Subsequently, a solution of 17 mL ethylene glycol with 7.2 mmol NH4F is added to the initial solution containing the chlorides and stirred for another 30 min. The resulting clear solution is then heated in a 250 mL Teflon lined autoclave (Berghof/America) with stirring for 24 h at 200 °C. The resulting nanoparticles may be isolated viacentrifugation using a mixture of distilled water and ethanol. TEM analysis showed the nanoparticles to be nearly spherical in shape with an approximate diameter of 18 nm (see Fig. S2 of the ESI† ). The PEI-capped nanoparticles crystallize in the cubic phase (α-NaYF4 phase) as was confirmed by XRD analysis.
An immortalized human carcinoma cell line derived from a HeLa cervical cancer was used in this work. The human HeLa carcinoma cells were routinely cultivated using Dulbecco modified Eagle medium (DMEM) containing 10% (vol/vol) fetal calf serum (FCS), 50 units/mL penicillin, 50 mg mL−1streptomycin. Cell cultures were performed at 37 °C in a humidified atmosphere containing 5% CO2. Cells were washed with phosphate-buffered saline (PBS) and afterwards incubated with the NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Yb3+NPs. The initial NPcolloidal solution (1 wt% in water) was diluted with PBS (1
Er3+, Yb3+NPs. The initial NPcolloidal solution (1 wt% in water) was diluted with PBS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 vol/vol). In order to explore the potential use of the non-functionalized NaYF4
20 vol/vol). In order to explore the potential use of the non-functionalized NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Yb3+NPs for intracellular activity studies, two different sets of HeLa cancer cells were prepared with incubation times of 1.5 and 3 h. After incubation, the cells were analyzed with a confocal fluorescence microscope, equipped with a femtosecond Ti:Sapphire laser (100 fs, 920 nm and 80 MHz repetition rate). The NIR laser beam was focused into the HeLa cells using a 50× microscope objective with a numerical aperture of 0.75. The simultaneous recording of the optical transmission image of the analyzed cell allowed us to precisely know the position of both the HeLa cell and the excitation spot. The two-photon visible fluorescence generated by the NPs was collected by the same 50× microscope objective and, after passing through a confocal aperture, it was focused into a fiber connected to a high-resolution spectrometer.
Er3+, Yb3+NPs for intracellular activity studies, two different sets of HeLa cancer cells were prepared with incubation times of 1.5 and 3 h. After incubation, the cells were analyzed with a confocal fluorescence microscope, equipped with a femtosecond Ti:Sapphire laser (100 fs, 920 nm and 80 MHz repetition rate). The NIR laser beam was focused into the HeLa cells using a 50× microscope objective with a numerical aperture of 0.75. The simultaneous recording of the optical transmission image of the analyzed cell allowed us to precisely know the position of both the HeLa cell and the excitation spot. The two-photon visible fluorescence generated by the NPs was collected by the same 50× microscope objective and, after passing through a confocal aperture, it was focused into a fiber connected to a high-resolution spectrometer.
Fig. 2(a) shows an optical transmission image of a HeLa cancer cell after 1.5 h incubation in the NP solution. Fig. 2(b) shows the upconversion emission spectra obtained when the fs laser pulses were focused “inside” and “outside” the cell (points labeled as A and B in Fig. 2(a), respectively). The red emission from the 4F9/2 → 4I15/2 transition of the Er3+ ions is clearly observed when the laser spot is located inside the cell. On the other hand no fluorescence at all (i.e. including any autofluorescence) is detected when the excitation beam is focused outside the cell, thus leading to a very high optical contrast. The high optical contrast obtained unequivocally denotes the incorporation of the NPs inside the HeLa cell as a consequence of an efficient endocytosis process. Indeed, this is supported by the observation of endocytosis vessels during the incubation procedure. Once the NPs have been incorporated in the cell, their spatial distribution will be affected by the intracellular dynamics that are associated with the intracellularnutrient transport. Therefore, the spatial distribution of the NPs (and hence the generated fluorescence intensity pattern) is expected to change with the incubation time. In order to verify this hypothesis, we have measured the spatial distribution of the NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Yb3+NPs visible fluorescence intensity for a single HeLa cell after incubation for 1.5 and 3 h. Results are shown in Fig. 3. The optical transmission images of single HeLa cells are shown in the top row ((a) and (b) for 1.5 and 3 h incubation times, respectively). The corresponding fluorescence images are shown in the bottom row ((c) and (d) for 1.5 and 3 h incubations, respectively). In both cases high-contrast fluorescence images have been obtained. It is clear that for short incubation times (1.5 h) the fluorescence intensity is more homogeneously distributed inside the cell, showing several maxima in the proximities of the cell membrane. On the other hand, for longer incubation times (3 h) the NaYF4
Er3+, Yb3+NPs visible fluorescence intensity for a single HeLa cell after incubation for 1.5 and 3 h. Results are shown in Fig. 3. The optical transmission images of single HeLa cells are shown in the top row ((a) and (b) for 1.5 and 3 h incubation times, respectively). The corresponding fluorescence images are shown in the bottom row ((c) and (d) for 1.5 and 3 h incubations, respectively). In both cases high-contrast fluorescence images have been obtained. It is clear that for short incubation times (1.5 h) the fluorescence intensity is more homogeneously distributed inside the cell, showing several maxima in the proximities of the cell membrane. On the other hand, for longer incubation times (3 h) the NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Yb3+NPs have been spatially re-distributed inside the cell, being mainly located in the proximity of the nucleus. We believe that this evident spatial re-distribution of the luminescent NPs is caused by the intracellular activity that is transporting the nutrients (and pseudo nutrients like the NPs) from the membrane to the Golgi apparatus (located close to nuclei). Results shown in Fig. 3 are, indeed, the first demonstration of the use of NaYF4
Er3+, Yb3+NPs have been spatially re-distributed inside the cell, being mainly located in the proximity of the nucleus. We believe that this evident spatial re-distribution of the luminescent NPs is caused by the intracellular activity that is transporting the nutrients (and pseudo nutrients like the NPs) from the membrane to the Golgi apparatus (located close to nuclei). Results shown in Fig. 3 are, indeed, the first demonstration of the use of NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Yb3+ upconverting NPs for monitoring intracellular dynamics. Finally, it should be mentioned that the incubated cells did not show any sign of toxicity during these experiments as was previously observed.35–37 Thus, these findings suggest that the NaYF4
Er3+, Yb3+ upconverting NPs for monitoring intracellular dynamics. Finally, it should be mentioned that the incubated cells did not show any sign of toxicity during these experiments as was previously observed.35–37 Thus, these findings suggest that the NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Yb3+NPs are highly suitable for intracellular dynamic studies requiring relatively long measuring times.
Er3+, Yb3+NPs are highly suitable for intracellular dynamic studies requiring relatively long measuring times.
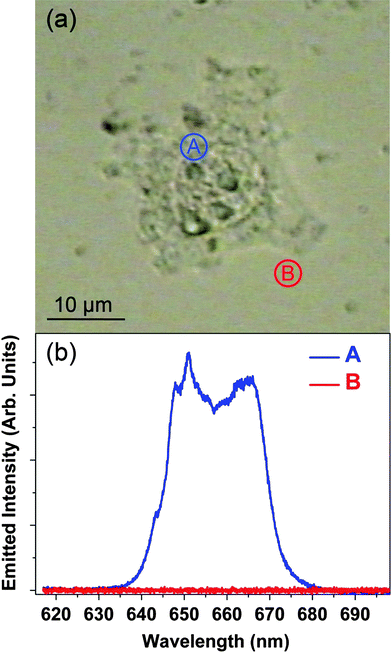 | ||
Fig. 2 (a) Optical transmission image of a single HeLa cancer cell after 1.5 h incubation in a water solution containing NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Yb3+NPs. (b) Visible two-photon micro-fluorescence spectra obtained when the fs NIR laser is focused inside and outside the HeLa cell (points A and B in the optical transmission image, respectively). Er3+, Yb3+NPs. (b) Visible two-photon micro-fluorescence spectra obtained when the fs NIR laser is focused inside and outside the HeLa cell (points A and B in the optical transmission image, respectively). | ||
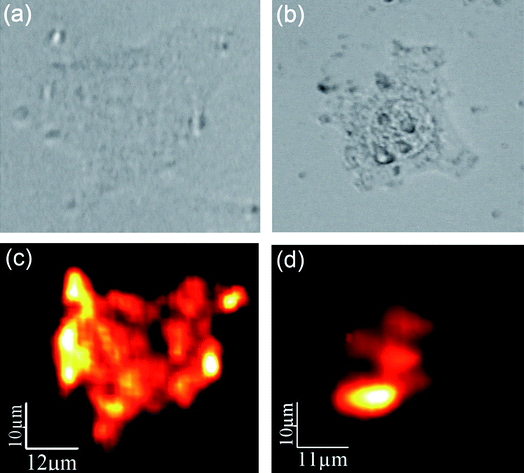 | ||
Fig. 3 Top – optical transmission images of two HeLa cells after incubation with NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Yb3+NPs during (a) 1.5 and (b) 3 h. Bottom – confocal fluorescence images of the same HeLa cells ((c) and (d) for 1.5 and 3 h incubations, respectively). Er3+, Yb3+NPs during (a) 1.5 and (b) 3 h. Bottom – confocal fluorescence images of the same HeLa cells ((c) and (d) for 1.5 and 3 h incubations, respectively). | ||
Conclusions
In summary, we have demonstrated that non-functionalized water soluble NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Yb3+ upconverting NPs can be easily incorporated into cancer cells by endocytosis. The very efficient NIR-to-visible fluorescence of the NPs after femtosecond laser excitation at 920 nm has been used to obtain intracellular images with an outstanding contrast and time stability. The single-cell images obtained after different incubation times clearly manifest a dynamical behavior of the NPs inside the cell as a consequence of the intracellular activity. For short incubation times, the NPs were homogeneously distributed inside the cell. For longer incubation times, and as consequence of the intracellular transportation of nutrients from the cellular membrane to the Golgi apparatus, the spatial distribution of the NPs becomes highly inhomogeneous. The results shown in this letter further the potential applications of NaYF4
Er3+, Yb3+ upconverting NPs can be easily incorporated into cancer cells by endocytosis. The very efficient NIR-to-visible fluorescence of the NPs after femtosecond laser excitation at 920 nm has been used to obtain intracellular images with an outstanding contrast and time stability. The single-cell images obtained after different incubation times clearly manifest a dynamical behavior of the NPs inside the cell as a consequence of the intracellular activity. For short incubation times, the NPs were homogeneously distributed inside the cell. For longer incubation times, and as consequence of the intracellular transportation of nutrients from the cellular membrane to the Golgi apparatus, the spatial distribution of the NPs becomes highly inhomogeneous. The results shown in this letter further the potential applications of NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Yb3+ upconverting NPs for static and dynamic bioimaging studies.
Er3+, Yb3+ upconverting NPs for static and dynamic bioimaging studies.
This work was supported by the Universidad Autónoma de Madrid ad Comunidad Autonoma de Madrid (Project No. CCG07-UAM/MAT-18611), by the Spanish Ministerio de Educacion y Ciencia (MAT 2007-64686) and by a Banco Santander-CEAL-UAM project. The authors also thank the Natural Sciences and Engineering Research Council (NSERC) of Canada and the Gouvernement du Québec, Ministère du Développement économique, de l'Innovation et de l'Exportation for funding.
References
- V. Ntziachristos, J. Ripoll, L. V. Wang and R. Weissleder, Nat. Biotechnol., 2005, 23, 313–320 CrossRef CAS.
- K. König, J. Microsc., 2000, 200, 83–104 CrossRef CAS.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, 3rd edn, Springer, New York, 2006 Search PubMed.
- Methods in Cellular Imaging, ed. A. Periasamy, Oxford University Press, New York, 2001 Search PubMed.
- W. Rudolph and M. Kempe, J. Mod. Optic., 1997, 44, 1617–1642 Search PubMed.
- B. R. Masters and P. T. C. So, Handbook of Biomedical Nonlinear Optical Microscopy, Oxford University Press, Oxford, 2008 Search PubMed.
- E. M. Sevick-Muraca, J. P. Houston and M. Gurfinkel, Curr. Opin. Chem. Biol., 2002, 6, 642–650 CrossRef CAS.
- D. W. Piston, Trends Cell Biol., 1999, 9, 66–69 CrossRef CAS.
- C. Xu, W. Zipfel, J. B. Shear, R. M. Williams and W. W. Webb, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 10763–10768 CrossRef CAS.
- I. Brigger, C. Dubernet and P. Couvreur, Adv. Drug Delivery Rev., 2002, 54, 631–651 CrossRef CAS.
- D. K. Chatterjee, L. S. Fong and Y. Zhang, Adv. Drug Delivery Rev., 2008, 60, 1627–1637 CrossRef CAS.
- X. Gao, Y. Cui, R. M. Levenson, L. W. K. Chung and S. Nie, Nat. Biotechnol., 2004, 22, 969–976 CrossRef CAS.
- J.-L. Li, L. Wang, X.-Y. Liu, Z.-P. Zhang, H.-C. Guo, W.-M. Liu and S.-H. Tang, Cancer Lett., 2009, 274, 319–326 CrossRef CAS.
- G. S. He, K.-T. Yong, Q. Zheng, Y. Sahoo, A. Baev, A. I. Ryasnyanskiy and P. N. Prasad, Opt. Express, 2007, 15, 12818–12833 CrossRef CAS.
- D. R. Larson, W. R. Zipfel, R. M. Williams, S. W. Clark, M. P. Bruchez, F. W. Wise and W. W. Webb, Science, 2003, 300, 1434–1436 CrossRef CAS.
- J. Park, A. Estrada, K. Sharp, K. Sang, J. A. Schwartz, D. K. Smith, C. Coleman, J. D. Payne, B. A. Korgel, A. K. Dunn and J. W. Tunnell, Opt. Express, 2008, 16, 1590–1599 CrossRef CAS.
- N. J. Durr, T. Larson, D. K. Smith, B. A. Korgel, K. Sokolov and A. Ben-Yakar, Nano Lett., 2007, 7, 941–945 CrossRef CAS.
- D.-S. Wang, F.-Y. Hsu and C.-W. Lin, Opt. Express, 2009, 17, 11350–11359 CrossRef CAS.
- F. Wang and X. Liu, Chem. Soc. Rev., 2009, 38, 976–989 RSC.
- D. K. Chatterjee, A. J. Rufaihah and Y. Zhang, Biomaterials, 2008, 29, 937–943 CrossRef CAS.
- S. A. Hilderbrand, F. Shao, C. Salthouse, U. Mahmood and R. Weissleder, Chem. Commun., 2009, 4188–4190 RSC.
- S. Jiang, Y. Zhang, K. M. Lim, E. K. W. Sim and L. Ye, Nanotechnology, 2009, 20, 155101 CrossRef.
- M. Wang, C.-C. Mi, W.-X. Wang, C.-H. Liu, Y.-F. Wu, Z.-R. Xu, C.-B. Mao and S.-K. Xu, ACS Nano, 2009, 3, 1580–1586 CrossRef CAS.
- T. Zako, H. Nagata, N. Terada, A. Utsumi, M. Sakono, M. Yohda, H. Ueda, K. Soga and M. Maeda, Biochem. Biophys. Res. Commun., 2009, 381, 54–58 CrossRef CAS.
- J.-C. Boyer, F. Vetrone, L. A. Cuccia and J. A. Capobianco, J. Am. Chem. Soc., 2006, 128, 7444–7445 CrossRef CAS.
- Z. Chen, H. Chen, H. Hu, M. Yu, F. Li, Q. Zhang, Z. Zhou, T. Yi and C. Huang, J. Am. Chem. Soc., 2008, 130, 3023–3029 CrossRef CAS.
- Z. Li, Y. Zhang and S. Jiang, Adv. Mater., 2008, 20, 4765–4769 CrossRef CAS.
- F. Wang and X. Liu, J. Am. Chem. Soc., 2008, 130, 5642–5643 CrossRef CAS.
- L. Wang, R. Yan, Z. Huo, L. Wang, J. Zeng, J. Bao, X. Wang, Q. Peng and Y. Li, Angew. Chem., Int. Ed., 2005, 44, 6054–6057 CrossRef CAS.
- F. Auzel, Chem. Rev., 2004, 104, 139–173 CrossRef CAS.
- R. Scheps, Prog. Quantum Electron., 1996, 20, 271–358 CrossRef CAS.
- A. P. Alivisatos, Science, 1996, 271, 933–937 CrossRef CAS.
- L. Tong, Q. Wei, A. Wei and J.-X. Cheng, Photochem. Photobiol., 2009, 85, 21–32 CrossRef CAS.
- H. Hu, L. Xiong, J. Zhou, F. Li, T. Cao and C. Huang, Chem.–Eur. J., 2009, 15, 3577–3584 CrossRef CAS.
- R. Abdul Jalil and Y. Zhang, Biomaterials, 2008, 29, 4122–4128 CrossRef.
- J. Shan, J. Chen, J. Meng, J. Collins, W. Soboyejo, J. S. Friedberg and Y. Ju, J. Appl. Phys., 2008, 104, 094308 CrossRef.
- F. Wang, D. K. Chatterjee, Z. Li, Y. Zhang, X. Fan and M. Wang, Nanotechnology, 2006, 17, 5786–5791 CrossRef CAS.
Footnote |
† Electronic supplementary information (ESI) available: Description and schematic representation of the upconversion process in lanthanide-doped nanoparticles, and TEM image of the NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Yb3+nanoparticles. See DOI: 10.1039/b9nr00236g Er3+, Yb3+nanoparticles. See DOI: 10.1039/b9nr00236g |
| This journal is © The Royal Society of Chemistry 2010 |
