Hydrophilic TiO2 porous spheres anchored on hydrophobic polypropylene membrane for wettability induced high photodegrading activities†
Fang
Niu‡
,
Le-Sheng
Zhang‡
,
Chao-Qiu
Chen‡
,
Wei
Li‡
,
Lin
Li
,
Wei-Guo
Song
* and
Lei
Jiang
*
Beijing National Laboratory for Molecular Sciences (BNLMS), Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, P. R. China. E-mail: wsong@iccas.ac.cn; jianglei@iccas.ac.cn; Fax: (+86) 10-62557908
First published on 8th June 2010
Abstract
TiO2 porous nanospheres on polypropylene (PP) films (TiO2/PP composite) are produced at ambient temperature. Particle/pore size match up is the key anchoring point to overcome the low affinity between hydrophilic materials and hydrophobic materials. With the hydrophilic TiO2 catalyst evenly dispersed on a hydrophobic surface, the aqueous solution will selectively skip the substrate and wet the catalysts. Such a wettability-induced smart system maximizes the degrading activity of the TiO2 catalyst. In photodegrading reactions, the resulting TiO2/PP composite film exhibits a 10 times higher activity in flow-type setup than the same TiO2 catalyst in a traditional batch-type setup.
Introduction
TiO2 material is of great research interest1–4 and is a widely used photocatalyst,5–8 especially in photodegradation of organic water contaminants.9,10 In general, organic molecules need to be close to the TiO2 surface to be degraded. However, in a conventional batch-type setup, the catalyst occupies only a small fraction of the solution. Most of the dye molecules in the bulk solution are far away from the catalyst, counting on random diffusion to reach the catalyst. In a flow-type setup, where an aqueous solution flows on the surface of a TiO2 thin film, the solution can wet the catalyst and form a thin layer of solution on the catalyst, ensuring that most of the organic molecules are close to the catalyst. Researchers have tried to attached TiO2 films on various substrates, including quartz,11 stainless steel,12 polymer.13 However, these methods focused more on system integrations than activity enhancements.Wettability plays a crucial role in chemistry at the solid/liquid interface.14–17 Wettability differences, gradients and switching provide intrinsic and elegant means to direct the flow of aqueous solution on substrates.18–21 For photodegradation of organic pollutants in aqueous solution, if hydrophilic TiO2 particles are evenly dispersed on a hydrophobic surface, the aqueous solution will skip the hydrophobic substrate and selectively wet the hydrophilic TiO2 catalysts; in other words, the organic molecules will find a photodegrading catalyst. Such a wettability-induced smart system can maximize the degrading activity of the TiO2 catalyst. However, no such system has been reported. The low affinity between hydrophilic particles with hydrophobic substrates may cause this.
In this work, we developed a simple but effective method to firmly anchor hydrophilic TiO2 porous nanospheres on hydrophobic polypropylene (PP) films at ambient temperature, and used it in a flow-type setup for photodegradation (Scheme 1). The resulting TiO2/PP composite film exhibits a 10 times higher activity in the flow-type setup than the same TiO2 catalyst in the batch-type setup. Dye molecules and other organic molecules can also be degraded to a deeper extent than in a batch-type reactor.
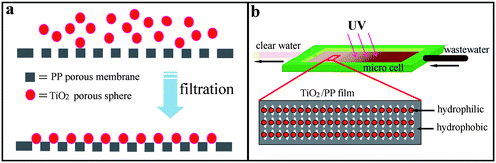 | ||
| Scheme 1 (a) Anchoring TiO2 porous nanospheres on porous PP membrane. (b) Experimental setup for continuous photodegrading process. | ||
Experimental
Preparation of TiO2 porous nanospheres
The TiO2 porous nanosphere was prepared according to the reported method by Zhong et al. with minor modifications.22 In a typical procedure, 0.4 ml of tetrabutyl titanate was added into 10 ml of ethylene glycol and then was magnetically stirred for 8 h at room temperature. Then, the tetrabutyl titanate–ethylene glycol colloid was poured into a mixture of 2.7 ml of water and 170 ml of acetone and was vigorous stirred for one hour. The white precipitate (TiO2 precursor, ESI†, Fig. S1) was gathered by centrifugation, followed by washing with ethanol and water five times. The obtained TiO2 precursor was dispersed into 30 ml of water and was heated to reflux under stirring. After one hour of refluxing, the obtained TiO2 porous nanospheres were gathered by centrifugation, followed by washing with ethanol five times and drying at 60 °C for further usage.Preparation of TiO2/PP composite thin film
In a typical procedure, 2.9 mg of as-prepared TiO2 porous nanospheres was ultrasonically dispersed into 1 L of ethanol to form a TiO2 colloidal solution. Then the TiO2 colloidal solution was filtrated through PP porous thin film under vacuum to form a TiO2/PP composite membrane, as shown in Scheme 1a. Various amounts of TiO2 porous nanospheres were deposited on the PP porous thin film via controlling the volume of TiO2 colloidal solution. Four samples with 0.0029, 0.058, 0.145, and 0.29 mg of TiO2 porous nanospheres on one piece of polypropylene film (40 mm × 16 mm) were obtained, respectively.Characterization
The TiO2 porous nanospheres, PP membrane, and TiO2/PP composites thin film were characterized by scan electron microscopy (SEM; Hitachi S-4300F), transmission electron microscopy (TEM; JEOL 1011), and X-ray diffraction (XRD; D/max-2500).Photodegrading activity test of TiO2/PP composite thin film
We tested the photodegrading activity of the TiO2/PP composite film for aqueous solution of dye molecules using continuous flow unit. As shown in Scheme 1b, a piece of TiO2/PP composite thin film was settled flat at the bottom of a Teflon reactor chamber, and then Congo Red aqueous solution (100 mg L−1) was pumped through the reactor chamber at a certain flow rate with UV irradiation (340 mw cm−2, obtained from a 400-W high pressure mercury lamp with a wavelength range from 350 nm to 450 nm). Along the flow direction, the red color of the solution becomes lighter due to the continuous degrading of the Congo Red by TiO2 nanoparticles on the TiO2/PP composite thin film. After 60 min of flow when the flow and degrading reaction become steady, the UV light was turned off to allow a snapshot of the chamber. The exiting solution was collected for 1 h at a flow rate of 5 ml h−1 to measure the Congo Red concentration with UV-Vis spectrometer (UV-1601, Shimadzu). Besides Congo Red, Methylene Blue, Rhodamine B, and several other organic molecule solutions were also photodegraded by the TiO2/PP composite membrane with the same procedure.The PP porous membrane does not show any activity for the photodegradation of Congo Red in our control experiments, and no adsorption of dye molecules is observed. The pure PP membrane is still white after flow experiments.
Photodegrading activity test of TiO2 porous nanospheres
We also tested the photodegrading activity of the pure TiO2 porous nanospheres for Congo Red aqueous solution. In a typical process, 0.29 mg, 1.45 mg, and 2.9 mg of TiO2 porous nanospheres was dispersed into 5 ml of Congo Red aqueous solution (100 mg L−1) with vigorous stirring. The mixture was photodegraded for 1 h under UV illumination and was analyzed with UV-Vis spectrometer to measure the Congo Red concentration.Quantitatively analysis of the photodegrading results
A photo processing program is used to quantitatively measure the photodegrading results in our experiments. The photos of the dye solutions under different flow rate were converted into grayscale images, and the grayness of the image at any point was obtained using the software. From the grayscale image, five evenly spaced points were chosen and the grayness of each points was obtained (the grayness of black was 256 and white was 0). The grayness at the starting point corresponds to the initial dye solution's concentration, while the grayness at the fifth point corresponds to the concentration of the exiting solution. Assuming a linear relation between the grayness and the dye concentration, the concentration of the dye solution along the flow course can then be plotted.Results and discussion
The key of this method is the porous PP membrane with pore channels that are perpendicular to the PP membrane surface. Fig. 1a shows the SEM image of PP membrane with relatively uniform pores of about 200 nm in length and 40 nm in width. Fig. 1b is the TEM image of the TiO2 porous nanospheres with a diameter of about 70 nm and is composed of nanoparticles of ca. 10 nm with a pure anatase phase (ESI,† Fig. S2). As illustrated in Scheme 1a, such particle/pore size fitting provides a nanoscale host/guest-type anchoring point for TiO2 porous nanospheres to be firmly attached to the PP membrane. By forcing a TiO2 porous nanosphere ethanol suspension through the pores of the PP membrane, TiO2 porous nanospheres are forced to insert partially but tightly into the pore mouth and stick there, forming TiO2/PP composites. Four TiO2/PP composites, with 0.0029, 0.058, 0.145, and 0.29 mg of TiO2 loading on a piece of PP film (40 mm × 16 mm), respectively, are obtained.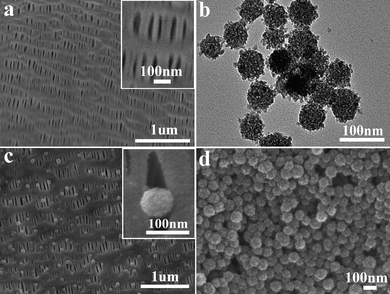 | ||
| Fig. 1 (a) SEM image of porous PP membrane; (b) TEM image of TiO2 porous nanospheres; SEM images of TiO2/PP composite with (c) 0.0029 mg and (d) 0.29 mg of TiO2 loading. The inserts in a and c are high resolution SEM images of the pores on the PP and TiO2/PP composites. | ||
Fig. 1c and 1d show the SEM images of TiO2/PP composite film with 0.0029 mg and 0.29 mg of TiO2 loading, respectively. TiO2 porous nanospheres in Fig. 1c are totally monodispersed and occupy most of the pores on PP membrane. The inset of Fig. 1c shows the anchoring of TiO2 particles at the pore mouth. With an increasing amount of TiO2 spheres, a TiO2 film is formed on the TiO2/PP composite (Fig. 1d).
At lower loadings, when there are enough holes for every particle, the TiO2 nanoparticles will find the holes, forming dispersed structures, as shown in Fig. 1c. There is a limitation to how much TiO2 nanospheres can be stuck into the holes. Beyond this, TiO2 nanospheres tend to stick to the nanospheres that are stuck into the holes, forming a thin-film-like TiO2 layer. The binding between TiO2 nanospheres is quite strong. Thus, even at higher loadings, the composite structures are quite durable. In our study, the highest loading is about 5 mg on one piece of PP membrane (40 mm × 16 mm).
The photodegrading activity of TiO2/PP composite membrane was tested using a home made continuous flow reactor as depicted in Scheme 1b. We first tested the photodegrading activity of the TiO2/PP composite film for Congo Red aqueous solution. After 60 min of flow when the flow and degrading reaction became steady, the UV light was turned off and snapshots of the chamber were taken to record the color profile along the flow course as shown in Fig. 2. Under a flow rate of 5 ml h−1, the four samples exhibited increasing photodegrading activity with higher TiO2 loadings. Fig. 2a–2d show the photos of Congo Red aqueous solution (100 mg L−1) degraded under UV irradiation at a flow rate of 5 ml h−1 by a TiO2/PP composite thin film deposited with 0.0029 mg, 0.058 mg, 0.145 mg, and 0.29 mg of TiO2 porous nanospheres, respectively. Along the flow direction, the red color of the solution has faded, but is still red in Fig. 2a. The color gradient become more and more evident from Fig. 2b to 2d, indicating an increasing photodegrading activity. Higher TiO2 porous nanosphere loading leads to a higher photodegrading activity. However, there is a limitation for the TiO2 loading, i.e. 1 mg on one piece of PP membrane (40 mm × 16 mm). Beyond 1 mg loading, the activity increase is not significant.
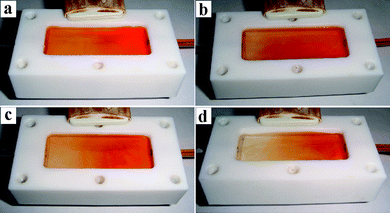 | ||
| Fig. 2 Photos of Congo Red aqueous solution (100 mg L−1) under UV irradiation at a flow rate of 5 ml h−1 on a TiO2/PP composite membrane with: (a) 0.0029 mg; (b) 0.058 mg; (c) 0.145 mg; and (d) 0.29 mg of TiO2 porous nanospheres loading, respectively. The solutions flow from right-to-left. | ||
The color of the Congo Red solution is red at the entrance and colorless before the exit in Fig. 2d, suggesting a complete photodegradation of the dye molecules and a high activity of the TiO2/PP composite film on which 0.29 mg of TiO2 porous nanospheres was deposited. The TiO2/PP composite thin film with 0.29 mg of TiO2 nanosphere loading was tested ten times with a total flow time of 60 h at a flow rate of 5 ml h−1. The photodegrading activity remained the same in all 10 runs.
The photodegrading activity of the TiO2/PP composite thin film with 0.29 mg of TiO2 under different flow rates was also tested using the continuous flow unit. Fig. 3a shows the images of photodegrading Congo Red solution at a flow rate of 3 ml h−1. The Congo Red solution is visible near the entrance of the reactor, however, the red color quickly faded, and becomes colorless before the middle point of the reactor. As the flow rate of Congo Red solution increases to 4 ml h−1 and 5 ml h−1, the exiting solutions are still colorless, however, as shown in Fig. 3b and 3c, it takes longer flow courses for the Congo Red solution to become colorless at higher flow rate. When the flow rate increases to 6 ml h−1 and 7 ml h−1 (Fig. 3d and 3e), the exiting solutions are no longer colorless, though significantly lighter than the entering solution. In a control experiment, a pure PP membrane was tested. As shown in Fig. 3f, there is no photodegrading activity by the PP membrane for Congo Red aqueous solution. The same red solution was observed throughout the reactor.
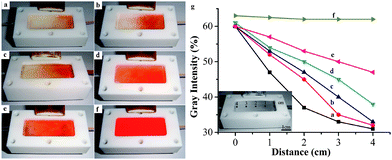 | ||
| Fig. 3 Photos of Congo Red aqueous solution (100 mg L−1) degraded by TiO2/PP composite thin film (deposited with 0.29 mg of TiO2 porous nanospheres) under UV irradiation at a flow rate of (a) 3 ml h−1, (b) 4 ml h−1, (c) 5 ml h−1, (d) 6 ml h−1, and (e) 7 ml h−1 in the Teflon micro cell, respectively. (f) Photo of Congo Red aqueous solution with polypropylene membrane; (g) the concentration profiles of Congo Red solutions along the flow direction with different flow rates. Inset in g is the grayscale image of c to determine the concentration profiles. | ||
To measure quantitatively the photodegrading results in these experiments, the images in Fig. 3 were treated by a photoprocessing software to convert them into grayscale images, and the grayness of the image at any point was obtained by the software. For example, the inset of the Fig. 3g is a grayscale image converted from Fig. 3c. From this grayscale image, five evenly spaced points were chosen and the grayness of each point was obtained and the concentration of the Congo Red solution along the flow course can then be plotted, as shown in Fig. 3g. Similarly, all images in Fig. 3a–3f are treated and the corresponding concentration vs. flow course plots are obtained.
Besides Congo Red, Methylene Blue and Rhodamine B can also be photodegraded by TiO2/PP composite thin film as shown in the ESI, Fig. S3.† Fig. S3c shows the photodegrading image for Methylene Blue solution (100 mg L−1) using TiO2/PP composite thin film with a flow rate of 5 ml h−1, which results in complete degradation of Methylene Blue at the end of the reactor. The flow rate of Methylene Blue solution at 3 ml h−1, 4 ml h−1, 6 ml h−1, 7 ml h−1, and 8 ml h−1 were also tested, and the photo images as well as the quantitative plots are shown in the ESI Fig. S3 and S5.† Similar results from the photodegradation of Rhodamine B solution (100 mg L−1) using TiO2/PP composite thin film were observed (See ESI,† Fig. S4 and S6).
In a flow-type reaction when the TiO2/PP composite membrane was used at a flow rate of 5 ml h−1 under UV irradiation, the exiting solution was collected during a one hour period (so that 5 ml of solution was collected within one hour). Several control experiments were carried out to compare the results of this method with other batch-type photodegrading methods. In one set of control experiments, 0.29 mg, 1.45 mg and 2.9 mg of TiO2 porous nanospheres, respectively, were used for batch-type photodegrading reactions. The same TiO2 porous nanospheres were mixed with 5 ml of Congo Red solution (100 mg L−1) for photodegradation with vigorous stirring under the same UV irradiation. After 1 h of UV irradiation, the Congo Red solution was collected by filtration and was analyzed by a UV-Vis spectrometer to measure the Congo Red concentration.
Fig. 4a shows the photos of Congo Red (100 mg L−1) aqueous solutions after various control experiments. The corresponding UV-Vis spectra of these solutions in Fig. 4a are shown in Fig. 4b. The red color of the solution becomes lighter with increasing TiO2 amount used (vials 2, 3, and 4) in the batch-type reactions. TiO2/PP composite in flow-type setup is about 10 times more active than those in a batch-type setup, as 10 times the TiO2 porous nanospheres are needed in the batch-type reaction to produce the same colorless solution (vials 4 and 5).
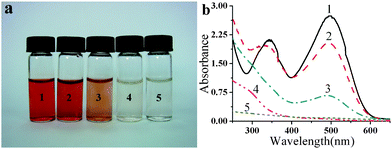 | ||
| Fig. 4 (a) Photos of Congo Red aqueous solution with an initial concentration of 100 mg L−1. (1) before photodegrading; (2)–(4) photodegraded by 0.29 mg, 1.45 mg, 2.9 mg of TiO2 porous nanospheres in the batch-type setup, respectively; and (5) photodegraded by TiO2/PP composite membrane with 0.29 mg of TiO2 loading using the flow-type setup with a flow rate of 5 ml h−1. (b) Corresponding UV-Vis absorption spectra of Congo Red aqueous solutions in (a). | ||
More importantly, as shown in Fig. 4b, the UV-Vis spectrum of the Congo Red solution treated by TiO2/PP composite membrane shows no absorbance in the entire UV and visible region (5 in Fig. 4b), while for batch-type control experiments, a significant adsorption remains at far UV region with 10 times more TiO2 catalyst (4 in Fig. 4b). Thus TiO2/PP composite film is not only highly active, but also lead to complete degrading.
This is due to the selective wetting of aqueous solution on the TiO2 catalyst induced by the wettability design of the TiO2/PP composite, i.e. hydrophilic TiO2 on hydrophobic PP membrane (see the ESI,†, Fig. S7). The flow-type reactor is crucial in the photodegrading activity. In a flow-type reactor, only a small amount of aqueous solution is on the composite membrane at any time during the reaction. Thus, the distribution of the aqueous solution is controlled by the wettability difference of the PP and TiO2. During the flow, aqueous solutions selectively wet the TiO2 porous nanospheres only, enabling most dye molecules to be in direct contact with the active site of the TiO2 for complete degrading. This prompts us to test its ability in photodegradation of some permanent organic pollutants (POPs). As shown in Fig. 5, chlorobenzene and naphthyl amine can be mostly photodegraded by TiO2/PP composite membrane. This provides a new route for POP ablation.
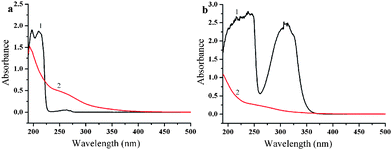 | ||
| Fig. 5 UV-Vis absorption spectra of (a) chlorobenzene and (b) naphthyl amine aqueous solution (50 mg L−1) before (1) and after (2) photodegraded by TiO2/PP composite membrane at a flow rate of 5 ml h−1 under UV irradiation. | ||
It will be interesting to see if the photodegrading activity can be changed by attaching the hydrophilic TiO2 porous spheres to a hydrophilic substrate. However, it is difficult to obtain a hydrophilic membrane with the similar holes to the PP membrane. Using normal non-porous hydrophilic substrate may not produce the same situation as the porous substrate.
To investigate the stability of the composite, we obtained an SEM image (see the ESI,† Fig. S8) of the TiO2/PP composite film (with 0.29 mg of TiO2 loading) after 60 h of photodegrading test at a flow rate of 8 ml h−1, and found no noticeable changes compared to the SEM image of fresh samples. In addition, we analyzed the exiting liquid by elemental analysis, and found no presence of titanium species. Thus we conclude that TiO2 nanosphere leakage did not occur.
Conclusions
In summary, we produced a TiO2/PP composite membrane using particle/pore size match up as the anchoring points, which resulted in hydrophilic catalysts on a hydrophobic substrate. The composite is highly active for complete photodegradation of organic dyes and even some POPs. The apparent photoactivity of this composite is about 10 times higher than the same catalyst in a batch-type reactor. This is a lab level conceptive study, and practical photocatalytic operation using this method is underway.Acknowledgements
We gratefully thank the National Natural Science Foundation of China (NSFC 50725207, 20821003), the National Basic Research Program of China (MOST 2007CB-936400 and 2009CB930400), and the Chinese Academy of Sciences for financial supports.References
- L. Liu, J.-S. Qian, B. Li, Y.-M. Cui, X.-F. Zhou, X.-F. Guo and W.-P. Ding, Chem. Commun., 2010, 46, 2402 RSC.
- Y.-M. Cui, L. Liu, B. Li, X.-F. Zhou and N.-P. Xu, J. Phys. Chem. C, 2010, 114, 2434 CrossRef CAS.
- L. Liu, Y.-M. Cui, B. Li, X.-F. Zhou and W.-P. Ding, Appl. Surf. Sci., 2010, 256, 2596 CrossRef CAS.
- J. H. Park, S. Y. Jung, R. Kim, N.-G. Park, J. Kim and S.-S. Lee, J. Power Sources, 2009, 194, 574 CrossRef CAS.
- M. Zhang, Q. Wang, C.-C. Chen, L. Zang, W.-H. Ma and J.-C. Zhao, Angew. Chem., Int. Ed., 2009, 48, 6081 CrossRef CAS.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37 CAS.
- F. E. Osterloh, Chem. Mater., 2008, 20, 35 CrossRef CAS.
- A. Fujishima, X. Zhang and D. A. Tryk, Surf. Sci. Rep., 2008, 63, 515 CrossRef CAS.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269 CrossRef CAS.
- A. L. Linsebigler, G.-Q. Lu and J. T. Yates, Chem. Rev., 1995, 95, 735 CrossRef CAS.
- J.-G. Yu, H.-G. Yu, B. Cheng, X.-J. Zhao, J. C. Yu and W.-K. Ho, J. Phys. Chem. B, 2003, 107, 13871 CrossRef CAS.
- J. C. Yu, W. Ho, J. Lin, H. Yip and P. K. Wong, Environ. Sci. Technol., 2003, 37, 2296 CrossRef CAS.
- A. Matsuda, Y. Kotani, T. Kogure, M. Tatsumisago and T. Minami, J. Am. Ceram. Soc., 2000, 83, 229 CrossRef CAS.
- F. Xia and L. Jiang, Adv. Mater., 2008, 20, 2842 CrossRef CAS.
- L. Jiang, Y. Zhao and J. Zhai, Angew. Chem., Int. Ed., 2004, 43, 4338 CrossRef CAS.
- R. Wang, K. Hashimoto and A. Fujishima, Nature, 1997, 388, 431 CrossRef CAS.
- R. Wang, K. Hashimoto, A. Fujishima, M. Chikuni, E. Kojima, A. Kitamura, M. Shimohigoshi and T. Watanabe, Adv. Mater., 1998, 10, 135 CrossRef CAS.
- L. Feng, Z.-Y. Zhang, Z.-H. Mai, Y.-M. Ma, B.-Q. Liu, L. Jiang and D.-B. Zhu, Angew. Chem., Int. Ed., 2004, 43, 2012 CrossRef CAS.
- P.-B. Wan, Y.-G. Jiang, Y.-P. Wang, Z.-Q. Wang and X. Zhang, Chem. Commun., 2008, 5710 RSC.
- X.-J. Feng and L. Jiang, Adv. Mater., 2006, 18, 3063 CrossRef CAS.
- F. Shi, Z.-Q. Wang and X. Zhang, Adv. Mater., 2005, 17, 1005 CrossRef CAS.
- L.-S. Zhong, J.-S. Hu, L.-J. Wan and W.-G. Song, Chem. Commun., 2008, 1184 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: XRD results for TiO2 porous nanospheres; photos of Methylene Blue, and Rhodamine B (100 mg L−1) aqueous solution degraded by TiO2/PP composite film under UV irradiation in the flow-type setup; quantitative plots of concentration profiles; contact angle of water droplets on TiO2 porous spheres and on PP membrane; SEM image of used TiO2/PP composite. See DOI: 10.1039/c0nr00182a |
| ‡ Also at the Graduate University of the Chinese Academy of Sciences. |
| This journal is © The Royal Society of Chemistry 2010 |
