Shape control of platinum and palladium nanoparticles for catalysis
Soshan
Cheong
,
John D.
Watt
and
Richard D.
Tilley
*
School of Chemical and Physical Sciences and The MacDiarmid Institute for Advanced Materials and Nanotechnology, Victoria University of Wellington, Wellington, 6012, New Zealand. E-mail: richard.tilley@vuw.ac.nz; Fax: +64 4 463 5241; Tel: +64 4 463 5335
First published on 6th August 2010
Abstract
Platinum and palladium are important catalysts for a wide variety of industrial processes. With the increasing demands of these materials, the development of high-performance catalysts is an important area of research, and as a result, shape control synthesis has become one of the leading research focuses. This minireview surveys the different approaches in solution-phase synthesis that have been successfully adopted for achieving shaped platinum and palladium nanoparticles that are enclosed with specific crystallographic facets. In addition, catalytic studies of the shaped nanoparticles are highlighted, in which promising results have been reported in terms of enhanced activity and selectivity. The future outlook discusses the aspects in synthesis and catalysis to be considered for the development of highly efficient and effective catalysts.
 Soshan Cheong | Soshan Cheong is currently a postdoctoral fellow at Victoria University of Wellington, New Zealand, from which she received her PhD in Chemistry (2010). During her PhD under the supervision of Dr Richard D. Tilley, she studied solution-phase synthesis and growth of nanoparticles for applications in catalysis and biomedicine. Her current research focuses on the controlled synthesis of catalytically active nanoparticles, with a particular emphasis on in situ characterisation and growth mechanisms of shaped nanoparticles. |
 John D. Watt | John Watt received a BSc in Chemistry (2004) from Massey University, Palmerston North, New Zealand. In 2009 he received the MacDiarmid Young Scientist of the Year (NZ) and Prime Ministers MacDiarmid Emerging Scientist Prizes (NZ) for his work on the shape control of complex palladium nanostructures. In 2010 he received his PhD under the supervision of Dr Richard Tilley. He is now working alongside Dr Richard Tilley as a project manager. His research interests include size and shape control of nanoparticles from the solution-phase focussing on palladium and other noble metals. |
 Richard D. Tilley | Richard Tilley is a Senior Lecturer in the School of Chemical and Physical Sciences at Victoria University of Wellington and has been since 2003. He is also a Principle Investigator and manager of the electron microscopes of The MacDiarmid Institute for Advanced Materials and Nanotechnlogy. He received his MChem from the University of Oxford and PhD in chemistry from the University of Cambridge. After which he was awarded a Toshiba Postdoctoral Fellowship and spent two years working in the nanotechnology group in Japan. Throughout his career he have worked on the liquid phase synthesis of nanocrystals with the aim of controlling properties, shape and structure. Current interests include catalytic metals, magnetic nanoparticles and silicon quantum dots. |
Introduction and background
The much larger surface-to-volume ratio of nanoparticles compared to their bulk counterparts has attracted a great deal of attention for catalytic applications.1–4 This is because much smaller amount of materials can be used, and higher catalytic activity can be achieved for catalysts of the same mass. Apart from the effects of size, the role of nanoparticle shape is also important in catalytic performance, which has been found to be highly dependent on the crystallographic planes that terminate the nanoparticle surface.5–7Both platinum and palladium are important catalysts for many industrial processes, in which they exhibit similar catalytic activities.8 Nanoparticles of platinum and palladium are heavily studied for various catalytic applications including hydrogenation reactions, carbon–carbon bond formation, and oxidation and reduction reactions in fuel cells.9–12 As the demands for precious metals such as platinum and palladium are growing, there has been a major focus on the development of high-performance catalysts.13 To achieve these demands research has been focused on improving the performance of these nanocatalysts by the formation of nanoparticles with fine control of both size and shape.
Over the years, solution-phase method has emerged as a highly versatile and powerful tool for size- and shape-controlled synthesis of nanoparticles of various materials.14–17 Currently, the size-controlled synthesis of spherical metal nanoparticles including those of platinum and palladium has been well established.18,19 Although different shapes have been reported, shape-controlled synthesis is much less developed compared to the control of size.20–22 In particular for platinum and palladium nanoparticles, the ability to precisely predict and control the nanoparticle shape that terminates with the desired crystallographic planes remains one of the main outstanding synthetic challenges.
Nevertheless, the development of shape-controlled synthesis has benefited much from the advancements of high-resolution transmission electron microscopy (TEM) and synchrotron-based techniques.23,24 These techniques allow for more precise and accurate structural characterisation, hence providing insightful information regarding nanoparticle growth. As a result of much intensive research, platinum and palladium nanoparticles of different morphologies have been reported in the actively growing area of shape-controlled synthesis.
In this minireview, we survey the recent development in solution-phase synthesis of platinum and palladium nanoparticles of different morphologies, and explore the latest progress in the area of shape-dependent catalytic studies. Discussions will highlight recent studies of controlled morphologies that have shown enhanced and interesting catalytic performance, which will lead to the development of efficient and effective nanocatalysts.
Nanoparticle morphology
Nanoparticles have high surface energies due to high surface-to-volume ratios at the nanoscale regime. As a result, nanoparticles in solution have a strong tendency to form into spherical or near-spherical polyhedral structures to minimise the total surface energy. Platinum and palladium adopt the highly symmetrical face-centred-cubic (fcc) crystal structure. Based on calculations, the predicted thermodynamic equilibrium shape for an fcc nanocrystal is a truncated octahedron bound by six {100} and eight {111} planes.25 The synthetic challenge is to make shapes that are different to these spherical or near-spherical polyhedral forms. Non-spherical shapes that have been successfully formed include highly faceted polyhedral structures enclosed by {100} or {111} facets such as cubes, tetrahedra and icosahedra, and 1-D rod-like, 2-D plate-like, and 3-D multiply branched and porous nanostructures.Nucleation and growth
Formation of nanoparticles in solution-phase synthesis involves (i) a nucleation event followed by (ii) growth stages in which solutes are consumed.16,26 The final nanoparticle morphology can be controlled by dictating the shape of nuclei and directing the growth of the nuclei and/or nanocrystals.Nuclei can take on a variety of shapes determined by the chemical potentials of the different crystallographic faces, which are in turn highly dependent on the reaction environment such as temperature and solute concentration.27 The nuclei shape can have a strong effect on the final nanocrystal shape, for example, through selected growth of high-energy crystal faces of the nuclei. An additional way in which the nuclei shape can aid the formation of non-spherical nanoparticles is through the introduction of twin planes.28,29 The fcc structure is highly symmetrical and twin planes in the nuclei can reduce the symmetry and aid the formation of non-spherical particles.
After the formation of nuclei, the subsequent growth stages also strongly govern the final morphology of the nanoparticles. Generally, nanoparticle growth can occur under two different regimes, either in a thermodynamically controlled or kinetically controlled growth regime.30 Thermodynamic growth is characterised by a sufficient supply of thermal energy and low flux of monomers.16 This often results in uniform growth of all crystal faces and the formation of spherical or near-spherical structures. In contrast, preferential and directional growth occurs under the non-equilibrium kinetic growth regime. The manipulation between the thermodynamic and the kinetic growth regimes is thus a critical factor in determining nanoparticle shape.
Shape control strategies
The general protocol for solution-phase synthesis of platinum and palladium nanoparticles involves the reduction and/or decomposition of a metal precursor in the presence of a surfactant or stabiliser.18,21,31–35 By variation of the type and relative concentration of the metal precursor, the reducing agent and stabiliser, as well as reaction conditions such as temperature and time, a myriad of variables are possible. Among these, a few have been found to be the most effective in the control of nanoparticle shape, through manipulating the nuclei shape and subsequent growth. These variables are explored in this section, and include (i) the type and amount of the stabilising agent used, (ii) the relative concentrations of the metal precursor and reducing agent, (iii) the variation of reaction temperature, and (iv) the introduction of seeds or foreign species.Growth controlled by the stabilising agent
In solution-phase synthesis, the stabilising molecules adsorb onto and desorb from the growing nanocrystal's surface in a dynamic fashion.36 Depending on the type of stabilisers used, the adsorption and desorption rates could be different on different crystallographic facets and affect nanocrystal growth. El-Sayed and co-workers were among the first to demonstrate the effect of stabilising agent on the shape control of single-crystal platinum nanoparticles.31 Tetrahedral nanocrystals were shown to form under high stabiliser concentration, which facilitated a stronger adsorption onto the {111} facets. This effectively reduced the surface energy and hence the growth rate of these faces, leading to the formation of {111}-terminated tetrahedra. Similar examples were also reported for the formation of platinum and palladium nanocubes enclosed by {100} facets, which were preferentially stabilised by the chosen stabilisers.21,33,37,38 In the above cases, growth is considered to be thermodynamically controlled due to nanocrystals being preferentially terminated by the stabilised or thermodynamically favoured facets.Additionally, by using a combination of stabilising agents with different functional groups that have different binding strengths such as acids and amines, the morphology of the resulting nanoparticles may be controlled. In a synthesis of platinum nanoparticles stabilised by adamantanecarboxylic acid (ACA) and hexadecylamine, Yang and co-workers demonstrated that by altering the ratio of ACA to platinum, nanoparticles of mono-, bi-, tri- and multipods could be obtained (as shown in Fig. 1), depending on the number of twin planes induced onto the growing nanocrystals.29,39
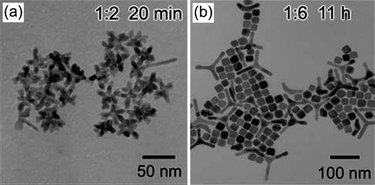 | ||
Fig. 1 Effect of stabiliser concentration on nanoparticle morphology. TEM images of platinum nanoparticles obtained at a Pt(II)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACA molar ratio of (a) 1 ACA molar ratio of (a) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and (b) 1 2 and (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6. (Reproduced from J. Phys. Chem. C, 2007, 111, 14312, copyright 2007 American Chemical Society.) 6. (Reproduced from J. Phys. Chem. C, 2007, 111, 14312, copyright 2007 American Chemical Society.) | ||
Recent studies by Watt et al. have also demonstrated that by varying the nature of the stabilising molecules, nuclei of the same shape could result in nanoparticles of distinctly different morphologies (see Fig. 2).40 Palladium nuclei of icosahedral shape were shown to retain the morphology during subsequent growth stages when only oleylamine was present as the stabiliser. In the presence of equal amounts of oleylamine and oleic acid, the nuclei were shown to grow into tripod nanoparticles. Formation of the latter was a result of destabilisation of the growing nanoparticle surface induced by oleic acid, which binds relatively weakly to palladium.16,41 This led to the increase in the rate of adatom addition thus allowing fast, crystallographically directional growth under a kinetically controlled growth regime. The fast growth of the tripod nanoparticles was later verified by in situ growth studies using synchrotron-based X-ray diffraction techniques.42 As the tripods continued to grow an increase in the growth rate was observed. The resulting kinetically driven, much faster growth that followed was shown to be entirely random and not crystallographically orientated, which then led to the formation of highly branched palladium nanostructures.
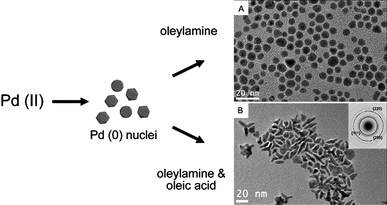 | ||
| Fig. 2 The use of stabilising agents with different functional groups for growth of different morphologies. Schematic and TEM images showing growth of polyhedral (A) and tripod (B) palladium nanoparticles governed by the stabilisation characteristics of the nanoparticles. (Reproduced from Adv. Mater., 2009, 21, 2288, copyright 2009 Wiley-VCH Verlag GmbH & Co.) | ||
Effects of reductant and metal precursor concentrations
In solution-phase synthesis, a mixture of different species is present, which includes the precursor, solvent, stabiliser and reducing agent. Among these, the effects of reductant in aqueous and polyol reduction syntheses have been frequently explored.7,21,30,33,43–45 For example, Xia and co-workers demonstrated that higher reductant concentration resulted in faster reduction rate for promoting directional growth of rod-like structures, and that the aspect ratios increased with the increase of reductant concentration (as shown in Fig. 3).38 The use of reductants with different reducing powers to regulate the reduction kinetics have also been reported in the synthesis of palladium nanoparticles of different morphologies, ranging from single-crystal and twinned polyhedra to rod-like and plate-like structures.21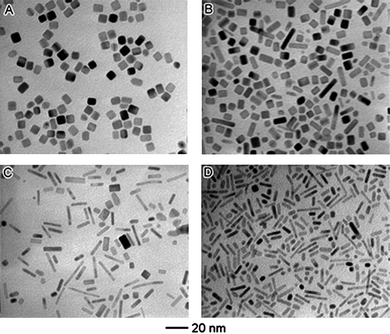 | ||
| Fig. 3 Effect of the type and concentration of reducing agent on the formation of 1-D nanoparticles. TEM images of palladium nanoparticles obtained from co-reduction of Na2PdCl4 by poly(vinyl pyrrolidone) and ethylene glycol (EG), with varying volume percent of EG: (A) 0%, (B) 9.1%, (C) 45.5%, and (D) 72.7%. (Reproduced from J. Am. Chem. Soc., 2007, 129, 3665, copyright 2007 American Chemical Society.) | ||
By adjusting the pH through addition of acid or base, different research groups have also managed to formulate reduction conditions that enabled the shape control of platinum and palladium nanoparticles.33,44,45 It has been demonstrated by Berhault et al. that highly reducing conditions could lead to a low monomer concentration in solution during the main part of nanoparticle growth, resulting in a thermodynamic control regime.30 Whereas under weakly reducing conditions, a high monomer concentration remained and growth stages become kinetically controlled, thus favouring directional growth yielding non-spherical shapes such as plate-like and branched structures. Different studies have also shown that reaction conditions pertaining to precursor and reductant at early stages could determine the growth behaviour of nanoparticles in the later stages.43,46,47
Depending on the rate of reaction, high precursor concentrations can result in high monomer concentrations and high chemical potentials, which are advantageous for the formation of branched nanostructures through directional growth under a kinetically controlled regime.36,48,49 For example, Yang and co-workers have demonstrated that branched platinum nanoparticles were obtained from a reductive decomposition synthesis employing high precursor concentration (as shown in Fig. 4).39,50 Bi- and tripod nanoparticles were reported to have grown from decahedrally and icosahedrally twinned nuclei, which were characteristic of a kinetically controlled regime. It was also shown that spherical nanoparticles predominantly formed in reaction with lower precursor concentration.
 | ||
| Fig. 4 Effect of increasing precursor concentration on the formation of branched nanoparticles. TEM images of platinum nanoparticles from reactions with different amounts of the precursor Pt(acac)2 used: (a) 50, (b) 100 and (c) 200 mg. All scale bars are 20 nm. (Reproduced from Nano Lett., 2005, 5, 885, copyright 2005 American Chemical Society.) | ||
Similarly, in a study reported by Ren and Tilley, thermodynamically favourable faceted cubic and triangular prismatic nanocrystals were obtained in a low-precursor concentration reaction.51,52 When the precursor concentration was increased 10-fold, branched and porous nanoparticles were obtained. An in situ growth study of the platinum nanoparticles later revealed that growth in the low-concentration solution occurred via a slow, layer-by-layer adatom addition to yield stable, faceted morphologies, which were characteristic of a thermodynamically controlled regime.46 In contrast, in the high-concentration reaction branched nanoparticles were formed at much greater rates under a kinetically controlled growth regime.
Shape control via temperature
Temperature is one of the main variables in decomposition reactions, as has been demonstrated by studies of different systems.17,53,54 Reaction temperature does not only affect the decomposition or reduction rate of the metal precursor, but also affects the growth kinetics by shifting the equilibrium established between the different species that co-exist in the solution. By varying the reaction temperature, different morphologies of platinum nanoparticles have been reported.37,55–57 For instance, Sun and co-workers demonstrated that near-spherical polyhedral, truncated cubic and cubic morphologies could be obtained at injection temperatures of 180, 160 and 120 °C, respectively (as shown in Fig. 5).56 It was reasoned that nucleation and growth processes were fast at high temperature and growth direction was not preferred, resulting in near-spherical polyhedral morphologies. In contrast, injection at lower temperature followed by a slow rate of heating facilitated a slow growth that led to the thermodynamic growth along the 〈111〉 over the 〈100〉 direction and hence the formation of larger cubes. | ||
| Fig. 5 Nanoparticle shape influenced by injection temperature. TEM images of (a) near-spherical polyhedral, (b) truncated cubic and (c) cubic platinum nanocrystals obtained from reactions with injection temperatures of 180, 160 and 120 °C, respectively. All scale bars in the insets are 1 nm. (Reproduced from Angew. Chem., Int. Ed., 2008, 47, 3588, copyright 2008 Wiley-VCH Verlag GmbH & Co.) | ||
The application of reaction temperature to promote directional growth of platinum nanoparticles leading to branched structures has also been demonstrated.51 Branched platinum nanoparticles with increasing number of branches were achieved at increased reaction temperatures, with tripods, octapods and multipods obtained at 40, 70 and 100 °C, respectively (see Fig. 6). The increase in the number of branches with the increase of temperature is an evidence of increasing growth kinetics, which has also been revealed by other studies of platinum nanoparticle synthesis.57–59
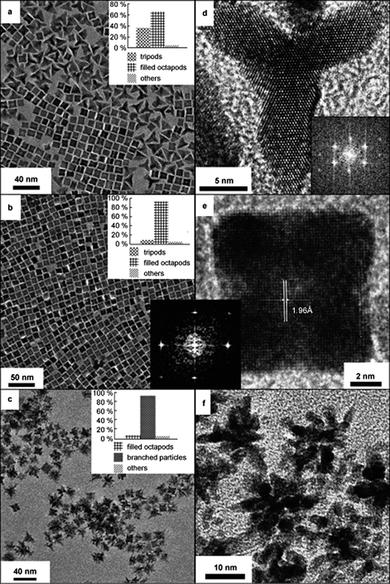 | ||
| Fig. 6 Effect of increasing reaction temperature on the formation of branched structures. TEM images of platinum nanoparticles synthesised at (a and d) 40, (b and e) 70 and (c and f) 100 °C, showing increase in number of branches with the increase of temperature. (Reproduced from Small, 2007, 3, 1508, copyright 2007 Wiley-VCH Verlag GmbH & Co.) | ||
The use of foreign ions and seed particles
There have been several studies reporting the use of foreign species, such as iron and silver ions, in trace amount (<5%), in the synthesis of platinum nanoparticles for shape control.32,50,56,60–62 For example, Xia and co-workers demonstrated that by introducing trace amount of iron ions to a polyol synthesis of platinum nanoparticles, the reduction kinetics could be altered to promote directional growth of nanowires or the formation of multi-octahedral nanocrystals.32,60It has been emphasised by different research groups that the presence of trace metallic ions was required to facilitate the nucleation and growth of cubic structures of platinum and palladium.47,56,61–63 Apart from nanocubes, other non-spherical nanostructures were also reported. For instance, rod-like and branched palladium nanoparticles were selectively prepared by Chen et al., with the addition of trace amount of copper ions (see Fig. 7).64 In a decomposition synthesis, Teng and Yang reported the use of Ag(acac) in trace amount to promote the formation of bi- and tripod platinum nanoparticles.50 It was discussed that Ag(acac) readily decomposed to form silver clusters, which catalysed the decomposition of Pt(acac)2. In the absence of Ag(acac), the decomposition could only occur at much higher temperature to produce spherical nanoparticles.
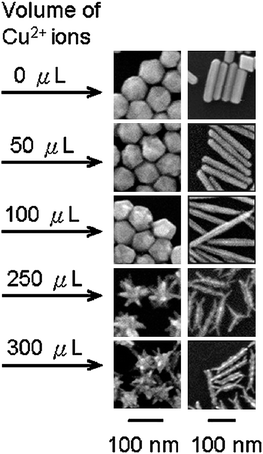 | ||
| Fig. 7 Effect of subtle increase in the concentration of foreign species (Cu2+ ions) on the formation of rod-like and branched nanostructures. SEM images of palladium nanoparticles showing formation of nanorods with increased aspect ratios and branched nanostructures under the influence of Cu2+ ions in trace amount. (Reproduced from J. Am. Chem. Soc., 2009, 131, 9114, copyright 2009 American Chemical Society.) | ||
The use of tiny nanocrystal seeds as nucleation centre for growth of larger nanocrystals is known for control of size and monodispersity of metal nanoparticles.65–68 For the purpose of shape control, this approach is relatively new, and much less explored for platinum and palladium, compared to those of other noble metals such as gold and silver.30 As nucleation is determined by the amount and physical nature (size and shape) of the seed particles added to the growth solution, the use of seeds with well-defined shapes may lead to morphologies different from those evolved from near-spherical nuclei or seeds.22,69 Using this approach, the synthesis of single-crystal platinum nanoparticles of porous-like, multi-armed structures was reported by Mahmoud et al., in which the nanocrystals were grown from seed particles of tetrahedral shape.70 Additionally, different studies have demonstrated that twinned and single-crystal palladium nanoparticles could be formed selectively, from seeds with and without the presence of twin planes, respectively.30,71,72
Probing growth mechanisms
As discussed, platinum and palladium nanoparticles of different morphologies have been achieved in solution-phase synthesis with different approaches. As a result, studies into shape-controlled growth have also gained increasing interest.19,73,74 One of the earliest studies discussing the shape-controlled growth mechanisms of platinum nanoparticles was that reported by El-Sayed and co-workers.75 It was proposed that the competition between monomer addition and stabiliser adsorption onto the {111} and {100} facets determined the nanoparticle morphology. Different mechanistic studies of shaped platinum and palladium nanoparticles have since developed, in which conclusions were mainly drawn from ex situ results collected over series of experiments.29,30,38,40,50More recently, kinetics studies based on an in situ or real time approach have shown potential as a useful tool to probe the shape-controlled growth mechanisms of nanoparticles.76 In a recent study, Alivisatos and co-workers reported an in situ TEM investigation of growing platinum nanoparticles, with experiments conducted in a liquid cell operated inside the TEM.77 The observations revealed that the nanoparticles grow by a combination of monomer attachment and particle coalescence, a route which was more complex than those envisioned previously. By combining in situ XRD and ex situ TEM observations, Cheong et al. have also reported a new mechanism for the formation of single-crystal porous platinum nanoparticles, which involved selective growth and etching processes that occurred simultaneously and at comparable rates.46 Besides, in situ XRD studies also confirmed the much faster growth rates for branched platinum and palladium nanoparticles under a kinetically controlled regime, in contrast to those of the respective faceted polyhedral nanocrystals.42,46
Catalytic studies of shaped nanoparticles
In catalytic studies of bulk crystals, it has been established that different facets and presence of kinks and steps could result in different activity and selectivity towards different reactions.78,79 This was found to be associated with the concentration of catalytically active atoms on the different types of surfaces.80,81 As nanoparticles of different shapes are enclosed by different facets, there are always different fractions of atoms located at different faces, corners, edges, and often defects. The catalytic properties are therefore expected to be different and interesting, in terms of both activity and selectivity. This section summarises some of the catalytic studies showing shape-dependent effects of platinum and palladium nanoparticles, which are grouped into (i) faceted polyhedral nanocrystals, (ii) 1-D nanorods and nanowires, and (iii) porous-like and branched nanostructures.Faceted polyhedral nanocrystals
Comparison studies of shape effects on catalytic activity were first reported by Narayanan and El-Sayed, in which cubic, truncated octahedral and tetrahedral platinum nanocrystals were investigated for the electron transfer reaction between [Fe(CN)6]3− and S2O32− ions.6,82–85 The average reaction rate was shown to increase exponentially with the increase of percentage of surface atoms on edges and corners of these faceted nanocrystals. It was found that the percentage of atoms located at corners and edges relative to the total surface atoms was highest for tetrahedral nanocrystals, followed by truncated octahedral and cubic nanocrystals, and hence the decrease in catalytic activities.84,86In catalytic studies for fuel cell applications, Sun and co-workers demonstrated that the specific activity of cubic platinum nanocrystals for oxygen reduction reaction was four times higher than those of truncated cubic and near-spherical polyhedral nanocrystals (see Fig. 8).56 Besides, twice the activity of the commercial spherical catalyst was achieved with the use of cubic nanocrystals.62 It was reported that the difference in catalytic activities was attributed to the much stronger bisulfate ion adsorption on Pt(111) than on Pt(100) faces, causing the reaction to be favourable on the latter. Other studies have also shown an enhanced oxidation rate of formic acid on Pd(100) than on Pd(111) and Pd(110).5,69 Other than faceted nanocrystals enclosed by low-index {100}, {111} and/or {110} facets, polyhedra of other shapes terminated with high-index facets are expected to exhibit interesting catalytic properties. For instance, platinum tetrahexahedral nanocrystals enclosed by high-index {210} and {310} facets were shown to exhibit an efficiency of almost 4 times more than that of the commercial Pt/C catalyst for ethanol electro-oxidation.87
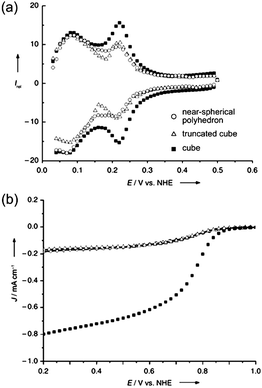 | ||
| Fig. 8 (a) Cyclic voltammograms (CVs) of platinum nanoparticles of near-spherical, truncated cubic and cubic shapes in 0.5 M H2SO4 at 10 mV s−1; much higher peak intensity of the CV of nanocubes indicates the dominance of Pt(100) planes. (b) Disk current densities at a rotation speed of 1600 rpm, showing enhanced activity exhibited by the nanocubes. The legend in (a) applies to both plots. (Reproduced from Angew. Chem., Int. Ed., 2008, 47, 3588, copyright 2008 Wiley-VCH Verlag GmbH & Co.) | ||
In terms of selectivity, hydrogenation reactions of benzene and pyrrole have been investigated by Somorjai and co-workers with platinum nanocrystals of cubic and cuboctahedral or near-spherical polyhedral shapes.7,37 While the different nanoparticles showed enhanced catalytic activity compared to bulk single crystals, cubic nanocrystals were shown to exhibit a higher selectivity towards cyclohexane and n-butylamine for benzene and pyrrole hydrogenation, respectively. As discussed, the above studies have thus shown the significance of shape and hence the terminating facets of the nanocatalysts in different reactions.
Nanorods and nanowires
The catalytic performance of 1-D platinum and palladium nanostructures such as nanorods and nanowires has received little attention compared to those of the polyhedral nanocrystals. Nevertheless, studies have demonstrated the potential of these nanocatalysts in different applications. For instance, palladium nanorods have been demonstrated as highly efficient and recyclable catalysts for Suzuki coupling reaction between phenylboronic acid and iodobenzene,64 and platinum nanowires have been shown to be catalytically active for CO oxidation at a lower temperature, as compared with catalysts prepared with spherical nanoparticles.59,88 In a recent study for methanol oxidation reaction, Xia and co-workers reported enhanced catalytic activities of platinum gauze covered with platinum nanowires grown along the 〈111〉, in comparison with catalyst prepared using plain platinum gauze.89 Similar catalysts prepared with platinum nanowires deposited onto carbon, as reported by Sun and Dodelet et al., also showed improved catalytic activity for oxygen reduction reaction compared with platinum nanoparticle catalyst.90,91Porous and branched nanostructures
Catalysts made of porous or branched nanostructures can combine the advantages of high surface area and more absorption sites for reactant molecules.58 This gives both improved catalytic performance and the opportunity to use these materials for gas adsorption and sensing.†As reported by Lee et al., porous platinum nanoparticles were observed to be more active than cubic and cuboctahedral nanocrystals for hydrogenation of ethylene (as shown in Fig. 9).33 Different studies have also shown enhanced catalytic activities exhibited by single-crystal porous-like platinum and palladium nanoparticles, compared to the respective nanocrystals of other faceted morphologies in different reactions, including electron transfer, oxidation and reduction reactions.58,60,70,92,93 These porous nanocrystals were shown to comprise groups of faceted nanocrystals in the same crystallographic orientation, resulting in the availability of more edges and corners as well as high-index sites for catalysis.
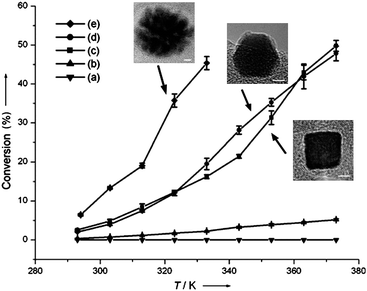 | ||
| Fig. 9 Comparison of catalytic activity for ethylene hydrogenation when using platinum (a) cuboctahedra of 9.1 nm, (b) cubes of 9.4 nm, (c) cubes of 13.4 nm, (d) cuboctahedra of 12.6 nm, and (e) porous nanoparticles of 19.3 nm. Nanoparticles of (a and b) were stabilised by PVP and (c–e) by TTAB. No treatment was performed to remove the stabilising agents. (Reproduced from Angew. Chem., Int. Ed., 2006, 45, 7824, copyright 2006 Wiley-VCH Verlag GmbH & Co.) | ||
In the case of palladium nanoparticles, catalytic studies of highly branched nanostructures have been recently reported for the hydrogenation of nitrobenzene.42 The nanocatalysts were highly selective and efficient, and no intermediate products were detected. It was revealed that the high activity was ascribed to the presence of high-index {113} facets on the branch edge, as a result of alternating {200} and {111} microfacets.
Considerations in catalytic applications
The above studies illustrate that platinum and palladium nanoparticles of well-defined morphologies are not only exhibiting enhanced catalytic activity, but also selectivity towards different reactions. Nevertheless, it is worth mentioned that studies have also shown stability and accessibility of the catalysts as the key issues to be considered. For example, in catalytic studies of platinum nanoparticles for Suzuki cross-coupling reaction that was conducted at high temperatures, shape changes from well-defined faceted nanocrystals to the less active spherical structures were observed by El-Sayed and co-workers, causing the catalysts to lose some of the activity.4,83In terms of accessibility for catalysis, the presence of residual stabilisers used in the synthesis could affect the catalytic performance of the nanoparticles. It is indeed a dilemma where strong binding stabilisers necessary for shaping the nanoparticles during synthesis could also be the barrier blocking or preventing the adsorption of reactants onto the catalyst surface, thereby greatly reducing the catalytic efficiency. Yang and co-workers have shown that the catalytic activity of platinum nanocrystals is much lower when stabilised by poly(vinyl pyrrolidone) (PVP), compared to those stabilised by tetradecyltrimethylammonium bromide (TTAB) (see Fig. 9).33 Choosing the optimum stabiliser is therefore critical to the catalytic applications of the nanoparticles.
Summary and outlook
The different approaches in solution-phase synthesis adopted for achieving morphology control of platinum and palladium nanoparticles include the variations of type and concentration of the metal precursor, stabilising and reducing agents, as well as reaction temperature, and also the use of seed particles and foreign species. In general, the different shape control techniques were applied to manipulate the nucleation and growth kinetics such that directional growth is promoted to selectively expose the desired crystallographic facets and hence the availability of catalytically active atoms. As catalysis is highly dependent on the surface nature of the catalyst, these shaped nanoparticles are potentially a new class of catalysts for enhanced catalytic performance, in terms of both activity and selectivity. Catalytic studies of different polyhedral, rod-like, and porous-like nanoparticles have also shown promising results in terms of improvements in activity and selectivity for a range of reactions.Nevertheless, there remain considerable challenges in the aspects of synthesis as well as catalysis. As the above shape control strategies have been mainly developed via an empirical approach, shape control is still not well understood. Although recent studies involving an in situ approach have emerged as a useful alternative tool for probing growth mechanisms, a combined experimental and theoretical study will be the more economical and practical approach. Future work in shape control synthesis will likely be focusing on finding a reliable and facile strategy for achieving precise control of the exposure of a specific set of crystallographic facets for the interest of improved catalytic properties. Furthermore, in order for the shaped nanocatalysts to be commercially viable, it is crucial to ensure the effects of stabilising agents and the stability of the nanoparticles during the catalytic reactions are properly considered.
Acknowledgements
R.D.T. acknowledges financial support from the MacDiarmid Institute. S.C. thanks the Royal Society of New Zealand for funding through Marsden Fund Contract IRL0602. R.D.T. and J.D.W. acknowledges the Australian Synchrotron, experiment number 723.Notes and references
- P. L. Freund and M. Spiro, J. Phys. Chem., 1985, 89, 1077–1087 CrossRef CAS.
- L. N. Lewis, Chem. Rev., 1993, 93, 2693–2730 CrossRef CAS.
- M. Moreno-Manas and R. Pleixats, Acc. Chem. Res., 2003, 36, 638–643 CrossRef CAS.
- R. Narayanan and M. A. El-Sayed, J. Phys. Chem. B, 2005, 109, 12663–12676 CrossRef CAS.
- M. Baldauf and D. M. Kolb, J. Phys. Chem., 1996, 100, 11375–11381 CrossRef CAS.
- R. Narayanan and M. A. El-Sayed, Nano Lett., 2004, 4, 1343–1348 CrossRef CAS.
- K. M. Bratlie, H. Lee, K. Komvopoulos, P. Yang and G. A. Somorjai, Nano Lett., 2007, 7, 3097–3101 CrossRef CAS.
- C. R. Henry, Surf. Sci. Rep., 1998, 31, 231–325 CrossRef.
- M. Arenz, K. J. J. Mayrhofer, V. Stamenkovic, B. B. Blizanac, T. Tomoyuki, P. N. Ross and N. M. Markovic, J. Am. Chem. Soc., 2005, 127, 6819–6829 CrossRef CAS.
- A. T. Bell, Science, 2003, 299, 1688–1691 CrossRef CAS.
- J. G. de Vries, Dalton Trans., 2006, 421–429 RSC.
- D. Astruc, Inorg. Chem., 2007, 46, 1884–1894 CrossRef CAS.
- B. C. Gates, G. W. Huber, C. L. Marshall, P. N. Ross, J. Siirola and Y. Wang, MRS Bull., 2008, 33, 429–435 CAS.
- V. F. Puntes, K. M. Krishnan and A. P. Alivisatos, Science, 2001, 291, 2115–2117 CrossRef CAS.
- J. Zhang and J. Fang, J. Am. Chem. Soc., 2009, 131, 18543–18547 CrossRef CAS.
- Y.-w. Jun, J.-H. Lee, J.-s. Choi and J. Cheon, J. Phys. Chem. B, 2005, 109, 14795–14806 CrossRef CAS.
- S. Kumar and T. Nann, Small, 2006, 2, 316–329 CrossRef CAS.
- T. Teranishi, M. Hosoe, T. Tanaka and M. Miyake, J. Phys. Chem. B, 1999, 103, 3818–3827 CrossRef CAS.
- J. Park, J. Joo, S. G. Kwon, Y. Jang and T. Hyeon, Angew. Chem., Int. Ed., 2007, 46, 4630–4660 CrossRef CAS.
- E. Ramirez, L. Eradès, K. Philippot, P. Lecante and B. Chaudret, Adv. Funct. Mater., 2007, 17, 2219–2228 CrossRef CAS.
- Y. Xiong and Y. Xia, Adv. Mater., 2007, 19, 3385–3391 CrossRef CAS.
- A. R. Tao, S. Habas and P. Yang, Small, 2008, 4, 310–325 CrossRef CAS.
- Z. L. Wang, J. Phys. Chem. B, 2000, 104, 1153–1175 CrossRef CAS.
- A. F. Craievich, Mater. Res., 2002, 5, 1–11 CAS.
- J. W. M. Frenken and P. Stoltze, Phys. Rev. Lett., 1999, 82, 3500–3503 CrossRef CAS.
- B. L. Cushing, V. L. Kolesnichenko and C. J. O'Connor, Chem. Rev., 2004, 104, 3839–3946.
- E. E. Finney and R. G. Finke, J. Colloid Interface Sci., 2008, 317, 351–374 CrossRef CAS.
- J. L. Elechiguerra, J. Reyes-Gasga and M. Jose-Yacaman, J. Mater. Chem., 2006, 16, 3906–3919 RSC.
- S. Maksimuk, X. Teng and H. Yang, J. Phys. Chem. C, 2007, 111, 14312–14319 CrossRef CAS.
- G. Berhault, M. Bausach, L. Bisson, L. Becerra, C. Thomazeau and D. Uzio, J. Phys. Chem. C, 2007, 111, 5915–5925 CrossRef CAS.
- T. S. Ahmandi, Z. L. Wang, T. C. Green, A. Henglein and M. A. El-Sayed, Science, 1996, 272, 1924–1926 CrossRef CAS.
- J. Chen, T. Herricks and Y. Xia, Angew. Chem., Int. Ed., 2005, 44, 2589–2592 CrossRef CAS.
- H. Lee, S. E. Habas, S. Kweskin, D. Butcher, G. A. Somorjai and P. Yang, Angew. Chem., Int. Ed., 2006, 45, 7824–7828 CrossRef CAS.
- S. W. Kim, J. Park, Y. Jang, Y. Chung, S. Hwang and T. Hyeon, Nano Lett., 2003, 3, 1289–1291 CrossRef CAS.
- V. Mazumder and S. Sun, J. Am. Chem. Soc., 2009, 131, 4588–4589 CrossRef CAS.
- Y. Yin and A. P. Alivisatos, Nature, 2005, 437, 664–670 CrossRef CAS.
- C.-K. Tsung, J. N. Kuhn, W. Huang, C. Aliaga, L.-I. Hung, G. A. Somorjai and P. Yang, J. Am. Chem. Soc., 2009, 131, 5816–5822 CrossRef CAS.
- Y. Xiong, H. Cai, B. J. Wiley, J. Wang, M. J. Kim and Y. Xia, J. Am. Chem. Soc., 2007, 129, 3665–3675 CrossRef CAS.
- S. Maksimuk, X. Teng and H. Yang, Phys. Chem. Chem. Phys., 2006, 8, 4660–4663 RSC.
- J. Watt, N. Young, S. Haigh, A. Kirkland and R. D. Tilley, Adv. Mater., 2009, 21, 2288–2293 CrossRef CAS.
- S. Ahrland, J. Chatt and N. R. Davies, Q. Rev. Chem. Soc., 1958, 12, 265–276 RSC.
- J. Watt, S. Cheong, M. F. Toney, B. Ingham, J. Cookson, P. T. Bishop and R. D. Tilley, ACS Nano, 2010, 4, 396–402 CrossRef CAS.
- Y. W. Lee, M. Kim and S. W. Han, Chem. Commun., 2010, 46, 1535–1537 RSC.
- Y. Piao, Y. Jang, M. Shokouhimehr, I. S. Lee and T. Hyeon, Small, 2007, 3, 255–260 CrossRef CAS.
- M. Nogami, R. Koike, R. Jalem, G. Kawamura, Y. Yang and Y. Sasaki, J. Phys. Chem. Lett., 2010, 1, 568–571 Search PubMed.
- S. Cheong, J. Watt, B. Ingham, M. F. Toney and R. D. Tilley, J. Am. Chem. Soc., 2009, 131, 14590–14595 CrossRef CAS.
- S. I. Lim, I. Ojea-Jimenez, M. Varon, E. Casals, J. Arbiol and V. Puntes, Nano Lett., 2010, 10, 964–973 CrossRef CAS.
- X. Peng, Adv. Mater., 2003, 15, 459–463 CrossRef CAS.
- S.-M. Lee, S.-N. Cho and J. Cheon, Adv. Mater., 2003, 15, 441–444 CrossRef CAS.
- X. Teng and H. Yang, Nano Lett., 2005, 5, 885–891 CrossRef CAS.
- J. Ren and R. D. Tilley, Small, 2007, 3, 1508–1512 CrossRef CAS.
- J. Ren and R. D. Tilley, J. Am. Chem. Soc., 2007, 129, 3287–3291 CrossRef CAS.
- Y.-w. Jun, J.-s. Choi and J. Cheon, Angew. Chem., Int. Ed., 2006, 45, 3414–3439 CrossRef CAS.
- L. Liu, Z. Zhuang, T. Xie, Y.-G. Wang, J. Li, Q. Peng and Y. Li, J. Am. Chem. Soc., 2009, 131, 16423–16429 CrossRef CAS.
- H.-T. Zhang, J. Ding and G.-M. Chow, Langmuir, 2008, 24, 375–378 CrossRef CAS.
- C. Wang, H. Daimon, O. Onodera, T. Koda and S. Sun, Angew. Chem., Int. Ed., 2008, 47, 3588–3591 CrossRef CAS.
- X. Zhong, Y. Feng, I. Lieberwirth and W. Knoll, Chem. Mater., 2006, 18, 2468–2471 CrossRef CAS.
- X. Teng, X. Liang, S. Maksimuk and H. Yang, Small, 2006, 2, 249–253 CrossRef CAS.
- D. Fenske, H. Borchert, J. Kehres, R. Kroger, J. Parisi and J. Kolny-Olesiak, Langmuir, 2008, 24, 9011–9016 CrossRef CAS.
- B. Lim, X. Lu, M. Jiang, P. H. C. Camargo, E. C. Cho, E. P. Lee and Y. Xia, Nano Lett., 2008, 8, 4043–4047 CrossRef CAS.
- H. Song, F. Kim, S. Connor, G. A. Somorjai and P. Yang, J. Phys. Chem. B, 2005, 109, 188–193 CrossRef CAS.
- C. Wang, H. Daimon, Y. Lee, J. Kim and S. Sun, J. Am. Chem. Soc., 2007, 129, 6974–6975 CrossRef CAS.
- M. Yamada, S. Kon and M. Miyake, Chem. Lett., 2005, 34, 1050–1051 CrossRef CAS.
- Y.-H. Chen, H.-H. Hung and M. H. Huang, J. Am. Chem. Soc., 2009, 131, 9114–9121 CrossRef CAS.
- H. Yu, P. C. Gibbons, K. F. Kelton and W. E. Buhro, J. Am. Chem. Soc., 2001, 123, 9198–9199 CrossRef CAS.
- N. R. Jana, L. Gearheart and C. J. Murphy, Langmuir, 2001, 17, 6782–6786 CrossRef CAS.
- A. Sánchez-Iglesias, I. Pastoriza-Santos, J. Pérez-Juste, B. Rodríguez-González, F. J. G. de Abajo and L. M. Liz-Marzán, Adv. Mater., 2006, 18, 2529–2534 CrossRef CAS.
- B. Hu, T. Wu, K. Ding, X. Zhou, T. Jiang and B. Han, J. Phys. Chem. C, 2010, 114, 3396–3400 CrossRef CAS.
- S. E. Habas, H. Lee, V. Radmilovic, G. A. Somorjai and P. Yang, Nat. Mater., 2007, 6, 692–697 CrossRef CAS.
- M. A. Mahmoud, C. E. Tabor, Y. Ding, Z. L. Wang and M. A. El-Sayed, J. Am. Chem. Soc., 2008, 130, 4590–4591 CrossRef CAS.
- S. Kinge and H. Bonnemann, Appl. Organomet. Chem., 2006, 20, 784–787 CrossRef CAS.
- B. Lim, M. Jiang, J. Tao, P. H. C. Camargo, Y. Zhu and Y. Xia, Adv. Funct. Mater., 2009, 19, 189–200 CrossRef CAS.
- C. Burda, X. Chen, R. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 105, 1025–1102 CrossRef CAS.
- M.-P. Pileni, J. Phys. Chem. C, 2007, 111, 9019–9038 CrossRef CAS.
- J. M. Petroski, Z. L. Wang, T. C. Green and M. A. El-Sayed, J. Phys. Chem. B, 1998, 102, 3316–3320 CrossRef CAS.
- M. F. Casula, Y.-w. Jun, D. J. Zaziski, E. M. Chan, A. Corrias and A. P. Alivisatos, J. Am. Chem. Soc., 2006, 128, 1675–1682 CrossRef CAS.
- H. Zheng, R. K. Smith, Y.-w. Jun, C. Kisielowski, U. Dahmen and A. P. Alivisatos, Science, 2009, 324, 1309–1312 CrossRef CAS.
- L. M. Falicov and G. A. Somorjai, Proc. Natl. Acad. Sci. U. S. A., 1985, 82, 2207–2211 CrossRef CAS.
- G. A. Somorjai, Introduction to Surface Chemistry and Catalysis, John Wiley & Sons, Inc., New York, 1994 Search PubMed.
- G. A. Somorjai and D. W. Blakely, Nature, 1975, 258, 580–583 CrossRef CAS.
- G. A. Somorjai, Chem. Rev., 1996, 96, 1223–1235 CrossRef CAS.
- R. Narayanan and M. A. El-Sayed, J. Phys. Chem. B, 2003, 107, 12416–12424 CrossRef CAS.
- R. Narayanan and M. A. El-Sayed, J. Am. Chem. Soc., 2004, 126, 7194–7195 CrossRef CAS.
- R. Narayanan and M. A. El-Sayed, J. Phys. Chem. B, 2004, 108, 5726–5733 CrossRef CAS.
- R. Narayanan and M. A. El-Sayed, Langmuir, 2005, 21, 2027–2033 CrossRef CAS.
- Z. Wang, T. S. Ahmad and M. A. El-Sayed, Surf. Sci., 1997, 380, 302–310 CrossRef CAS.
- N. Tian, Z.-Y. Zhou, S.-G. Sun, Y. Ding and Z. L. Wang, Science, 2007, 316, 732–735 CrossRef CAS.
- H. Borchert, D. Fenske, J. Kolny-Olesiak, J. Parisi, K. Al-Shamery and M. Baumer, Angew. Chem., Int. Ed., 2007, 46, 2923–2926 CrossRef CAS.
- E. P. Lee, Z. Peng, W. Chen, S. Chen, H. Yang and Y. Xia, ACS Nano, 2008, 2, 2167–2173 CrossRef CAS.
- S. Sun, D. Yang, D. Villers, G. Zhang, E. Sacher and J.-P. Dodelet, Adv. Mater., 2008, 20, 571–574 CrossRef CAS.
- S. Sun, F. Jaouen and J.-P. Dodelet, Adv. Mater., 2008, 20, 3900 CrossRef CAS.
- L. Wang and Y. Yamauchi, Chem. Mater., 2009, 21, 3562–3569 CrossRef CAS.
- M. Sanles-Sobrido, M. A. Correa-Duarte, S. Carregal-Romero, B. Rodríguez-González, R. A. Alvarez-Puebla, P. Herves and L. M. Liz-Marzán, Chem. Mater., 2009, 21, 1531–1535 CrossRef CAS.
- V. Berube, G. Radtke, M. Dresselhaus and G. Chen, Int. J. Energy Res., 2007, 31, 637–663 CrossRef CAS.
Footnote |
| † Synchrotron-based XRD experiments were conducted recently to investigate the hydrogen absorption properties of palladium nanoparticles in situ, at the Australia Synchrotron (AS) in Melbourne, Australia. The in situ XRD results showed that palladium hydride of the β-PdH phase was formed, which then became the predominant phase, when porous palladium nanostructures were exposed to a hydrogen atmosphere at 70 °C. This indicated the hydrogen absorption by the palladium nanoparticles. The desorption of hydrogen was observed when the diffraction peaks of β-PdH diminished and those of Pd grew again, at room temperature upon exposure to air. The porous palladium nanostructures thus showed great potential for the hydrogen economy. It should particularly be noted for the hydrogen desorption that occurred at room temperature, which is in stark contrast to most hydrides which must be heated to ∼200 °C for hydrogen release.94 |
| This journal is © The Royal Society of Chemistry 2010 |
