Room temperature synthesis and optical properties of small diameter (5 nm) ZnO nanorod arrays†
Seungho
Cho
,
Ji-Wook
Jang
,
Jae Sung
Lee
and
Kun-Hong
Lee
*
Department of Chemical Engineering, Pohang University of Science and Technology (POSTECH), San 31, Hyoja-Dong, Nam-Gu, Pohang, Gyungbuk, Korea 790-784. E-mail: ce20047@postech.ac.kr
First published on 10th August 2010
Abstract
We report a simple wet-chemical synthesis of ∼5 nm diameter ZnO nanorod arrays at room temperature (20 °C) and normal atmospheric pressure (1 atm) and their optical properties. They were single crystalline in nature, and grew in the [001] direction. These small diameter ZnO nanorod arrays can also be synthesized at 0 °C. Control experiments were also conducted. On the basis of the results, we propose a mechanism for the spontaneous growth of the small diameter ZnO structures. The optical properties of the 5 nm diameter ZnO nanorod arrays synthesized using this method were probed by UV-Visible diffuse reflectance spectroscopy. A clear blue-shift, relative to the absorption band from 50 nm diameter ZnO nanorod arrays, was attributed to the quantum confinement effects caused by the small nanocrystal size in the 5 nm diameter ZnO nanorods.
1. Introduction
Zinc oxide (ZnO) is a II–VI semiconductor with a wide direct band gap of 3.37 eV at room temperature and a high electron-hole binding energy of 60 meV. ZnO shows useful characteristics, such as a large piezoelectric constant, and modification of its electrical conductivity is easy. ZnO has been investigated intensively due to these excellent properties.1 In particular, one-dimensional (1D) ZnO nanostructures with diameters of less than 10 nm are expected to display novel and unique physical and chemical properties due to quantum confinement,2 and dimensional reduction of 1D nanostructures has been actively pursued. Previous investigations have focused on the syntheses of small diameter ZnO powders via homogeneous reactions in the presence of a surfactant.3–5 Direct synthesis of 1D nanostructures on a substrate is desirable for a variety of applications, such as field effect transistors,6 chemical sensors,7 field emitters,8 transparent conductors,9 ultraviolet light emitting devices,10 and photocatalytic processes.11 Reports of the direct synthesis of small diameter (less than 10 nm) 1D ZnO nanostructures on a substrate are relatively rare. Fang et al. grew thin (≤ 10 nm) ZnO nanofibers on a substrate using 95 °C hydrothermal reactions,2 and Yang et al. synthesized ZnO nanostructures (diameter or thickness < 10 nm) via an electrochemical route.12Development of total chemical syntheses at room temperature under normal atmospheric pressure is desirable for the purposes of mimicking natural mineralogical or biological processes,13,14 and for reducing the energy requirements for device fabrication. Methods for preparing very small diameter and highly crystalline ZnO nanorod arrays at room temperature and under normal atmospheric pressure are also desirable, because such conditions permit control of the band structures in the quantum confinement regime to provide building blocks for nanodevices and to increase the surface-to-volume (S/V) ratio, which increases the surface area available for catalytic applications of ZnO. Na2O2 solution is strong oxidant and can be used as oxygen agent, bleach, fungicides or disinfectants. Na2O2 aqueous solution has potential to be used in the fabrication of metal oxide nanostructure arrays using metal substrates. Recently, Liu and co-workers used Na2O2 aqueous solution to synthesize Cu(OH)2 and CuO nanoribbon arrays on the Cu foil with silver nitrate.15 In this paper, we report a method for fabricating 5 nm diameter ZnO nanorod arrays at room temperature (20 °C) and normal atmospheric pressure (1 atm) using Na2O2 aqueous solution. This method uses little energy and requires no complex experimental procedures or equipment, no surfactants, and no template. Interestingly, 5 nm diameter ZnO nanorod arrays can also be synthesized at 0 °C.
2. Experimental section
All chemicals used in this work were of analytical grade and were used without further purification. A 100 mL aqueous solution containing 0.2 M sodium peroxide (Na2O2, 28.0–30.0 wt%, Samchun) was prepared at room temperature (pH 13.9). A cleaned Zn foil (Nilaco, 99.5%, feature size 1.5 × 1.5 cm2, 0.25 mm thick) was incubated in the as-prepared solution at room temperature (20 °C) for 2 h. The substrate was washed by ultrasonication in deionized water for 1 min, after which it was dried in an oven for 12 h. The morphology, crystallinity, crystalline nature, chemical composition, and optical properties of the samples were observed using a field-emission scanning electron microscope (FESEM, JEOL JMS-7400F, operating at 10 keV), a high-resolution scanning transmission electron microscope (Cs corrected HR-STEM, JEOL JEM-2200FS with an energy-dispersive X-ray spectrometer (EDX), operated at 200 kV), X-ray photoelectron spectroscopy (XPS, VG Scientific, EscaLab 200iXL), and X-ray diffraction (XRD, Mac Science, M18XHF). For TEM observations, the synthesized nanorods were separated from the substrate using a knife and were dispersed in ethanol by ultrasonication. Several drops of the ethanol dispersion were introduced onto a Cu grid and dried in a vacuum oven at 60 °C for 12 h.3. Results and discussion
Fig. 1(a) and 1(b) display the XRD patterns of the Zn foil before and after reaction. Prior to the reaction, the XRD pattern of the original bare Zn foil (Fig. 1(a)) showed sharp peaks corresponding to Zn crystalline planes (JCPDS No. 04-0831). After the reaction, the XRD pattern showed additional peaks (Fig. 1(b)) that could be indexed as a hexagonal wurtzite ZnO structure with calculated lattice constants of a = 0.325 nm and c = 0.521 nm. These lattice constants were consistent with previously reported data (JCPDS No. 36-1451). Broadened diffraction peaks could be seen for ZnO crystals, indicating their small grain size. No diffraction peaks from other impurities were observed in the XRD patterns.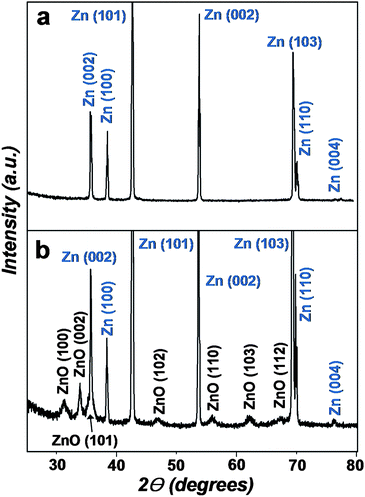 | ||
| Fig. 1 XRD patterns of the Zn foil (a) before and (b) after reaction in aqueous 0.2 M Na2O2 solution at room temperature for 2 h. | ||
Fig. 2(a) and 2(b) show SEM images of the surface of the substrate after the reaction. ZnO crystals with rod-like structures were uniformly distributed on the surface of the Zn foil. The length, diameter, and shape of the ZnO structures were highly uniform. These rod-like structures were, on average, 50 nm in length and 5 nm (±1.5 nm) in diameter. The structure of ZnO nanorods was analyzed under higher magnification using a high-resolution scanning transmission electron microscope (HR-STEM). Fig. 3(a) shows a TEM image of ZnO nanorods synthesized and detached from the substrate. An HR-TEM image (Fig. 3(b)) shows that ZnO nanorods were highly crystalline, with lattice spacings of 0.26 nm, corresponding to the distance between the (002) planes in the ZnO crystal lattice. All synthesized and characterized nanorods were single crystalline in nature, and the nanorods grew in the [001] direction.
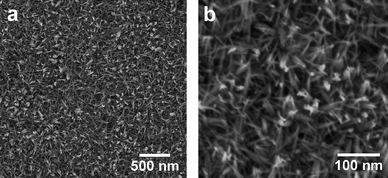 | ||
| Fig. 2 SEM images of the surface of the ZnO nanorod arrays synthesized by reaction of a Zn foil with an aqueous 0.2 M Na2O2 solution at room temperature for 2 h: (a) Low magnification. (b) High magnification. | ||
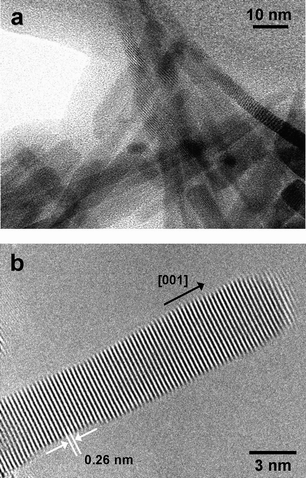 | ||
| Fig. 3 (a) TEM image of ZnO nanorods that had been detached from the substrate after reaction of a Zn foil with an aqueous 0.2 M Na2O2 solution at room temperature for 2 h. (b) HR-TEM image of a ZnO nanorod synthesized via reaction of a Zn foil with an aqueous 0.2 M Na2O2 solution at room temperature for 2 h. | ||
The composition of the ZnO nanorods was investigated using EDX and XPS. The EDX pattern (Figure S1 in the Electronic Supplementary Information (ESI))† indicated that the ZnO structures were composed of only Zn and O, because the Cu signal was attributed to the copper mesh used for HR-TEM. Quantitative analysis of the mean atomic ratio (Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O) in the ZnO structure yielded 0.502
O) in the ZnO structure yielded 0.502![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.498 (by EDX) and 0.504
0.498 (by EDX) and 0.504![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.496 (by XPS). No evidence of other impurities was found. ZnO nanorods had a nearly stoichiometric atomic ratio. These data also confirmed the high purity of the ZnO nanorods.
0.496 (by XPS). No evidence of other impurities was found. ZnO nanorods had a nearly stoichiometric atomic ratio. These data also confirmed the high purity of the ZnO nanorods.
The time-dependent evolution of nanorod morphology was characterized (Figure S2 in the ESI).† At early stages (10 min), nucleation occurred on the Zn substrate to form ZnO dots (Figure S2(a)).† After reacting for 30 min, short nanorods grew at the nucleation sites (Figure S2(b)).† At 2 h reaction time, 5 nm diameter ZnO nanorod arrays were synthesized (Figure S2(c)).† Even if the reaction time was extended to 6 h (Figure S2(d)),† ZnO nanorods had achieved nearly the same dimensions as those grown within 2 h. The temperature-dependence of ZnO nanorod array growth was characterized (Fig. 4). Interestingly, 5 nm diameter ZnO nanorod arrays could be synthesized at 0 °C. To the best of our knowledge, this is the lowest reported reaction temperature for ZnO nanorod synthesis. At 0 °C, the growth rate of ZnO crystals was significantly reduced. At 2 h reaction times, ZnO had nucleated on the substrate (Fig. 4(a)), and even at 6 h, only very short nanorods had formed (Fig. 4(b)). However, when the reaction time was extended to 24 h (Fig. 4(c)), arrays were obtained that were indistinguishable from the arrays grown within 2 h at room temperature. When the reaction temperature was increased to 95 °C (with a 2 h reaction time), the average diameter of ZnO nanorods increased, and the diameter variation also increased (Fig. 4(d)). Fig. 4(f) shows a TEM image of ZnO nanorods synthesized from the reaction at 0 °C for 24 h and detached from the substrate. An HR-TEM image (Fig. 4(f)) shows that ZnO nanorods which grew at 0 °C had single crystalline structures, with lattice spacings of 0.26 nm, corresponding to the interspacings of the (002) planes in the ZnO crystal lattice.
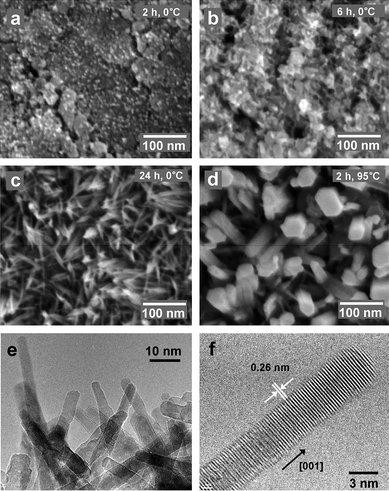 | ||
| Fig. 4 SEM images of the surface of the substrates after the reaction with the aqueous 0.2 M Na2O2 solution: (a) At 0 °C for 2 h; (b) At 0 °C for 6 h; (c) At 0 °C for 24 h; (d) At 95 °C for 2 h. (e) TEM image of ZnO nanorods that had been detached from the substrate after reaction of a Zn foil with an aqueous 0.2 M Na2O2 solution at 0 °C for 24 h. (f) HR-TEM image of a ZnO nanorod synthesized via reaction of a Zn foil with an aqueous 0.2 M Na2O2 solution at 0 °C for 24 h. | ||
When the Zn foil was immersed in an aqueous NaOH solution (pH 13.9) and maintained at room temperature, no ZnO nanorods grew on the surface (Figure S3(a): 2 h; Figure S3(b): 6 h; Figure S3(c): 24 h, in the ESI).† When the ZnO-nucleated Zn foil (prepared by reaction with 0.2 M Na2O2 at room temperature for 30 min (Figure S2(b))† was immersed in an aqueous NaOH solution (pH 13.9) and maintained at room temperature, no further ZnO crystal growth occurred (Figure S3(d): 2 h; Figure S3(e): 6 h; Figure S3(f): 24 h).† The nucleation and growth of ZnO crystals apparently did not occur even in the presence of ample hydroxyl ion anions (OH−) at room temperature.
Sodium peroxide hydrolyzes to give sodium hydroxide and hydrogen peroxide according to the reaction:
| Na2O2 + H2O → 2Na+ + 2OH− + H2O2 | (1) |
The hydrogen peroxide solution is unstable, and it decomposes into oxygen, providing an oxygen-rich environment. Several reactions may be involved in the formation of ZnO nanorods on a Zn surface in aqueous sodium peroxide:
| Zn(s) + H2O2 + 2OH− → ZnO2−2 + 2H2O | (2) |
| ZnO2−2 + H2O → ZnO + 2OH− | (3) |
In the NaOH case, the nucleation and growth of ZnO crystals did not occur as shown in Figure S3.† In contrast, hydrogen peroxide, donating oxygen readily, reacted with the Zn surface and hydroxyl anions in the sodium peroxide case. Eqn (2) describes the release of ZnO22− locally, which increases the concentration of this anion near the substrate surface. Once the concentration of ZnO22− ions reaches saturation, nucleation and subsequent growth of ZnO nanorods may occur on the Zn surfaces at room temperature viaeqn (3).16–18 This condition could not be created in the NaOH case. Therefore, we speculated that the local degree of saturation of ZnO22− ions near the substrate and the low reaction temperature were key factors for the formation of 5 nm ZnO nanorods.
Because the Bohr exciton radius of ZnO is 2.34 nm,19 a nanoscale entity of this material when approaching this size regime is expected to show quantum confinement effects. The exciton emission was blue-shifted for ZnO nanobelts or nanorods in which the smallest dimension was less than 10 nm.20,21 The optical properties of small diameter ZnO nanorod (average diameter: 5 nm) arrays synthesized using this method were probed by UV-Visible diffuse reflectance spectroscopy, as shown in Fig. 5. These properties were compared with those of 50 nm diameter ZnO nanorod arrays (SEM image: Figure S4 in the ESI).†Fig. 5(a) and 5(b) are the UV-Visible diffuse reflectance spectra of 5 nm diameter ZnO nanorod arrays synthesized at room temperature for 2 h and 5 nm diameter ZnO nanorod arrays synthesized at 0 °C for 24 h, respectively. A clear blue-shift, relative to the absorption bands from 50 nm diameter ZnO nanorod arrays (Fig. 5(c)), were attributed to the quantum confinement effects caused by the small nanocrystal size in the 5 nm diameter ZnO nanorods.
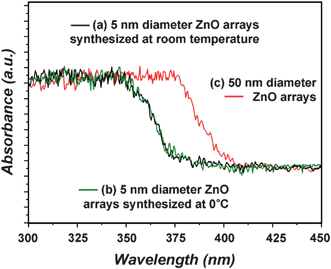 | ||
| Fig. 5 UV-Visible diffuse reflectance spectra: (a) 5 nm diameter ZnO nanorod arrays synthesized from the reaction of a Zn foil with an aqueous 0.2 M Na2O2 solution at room temperature for 2 h. (b) 5 nm diameter ZnO nanorod arrays synthesized from the reaction of a Zn foil with an aqueous 0.2 M Na2O2 solution at 0 °C for 24 h. (c) 50 nm diameter ZnO nanorod arrays synthesized from the 90 °C hydrothermal reaction of a Zn foil with an aqueous ammonia solution for 2 h. | ||
4. Conclusions
We have reported a very simple wet chemical synthesis of 5 nm diameter ZnO nanorod arrays at room temperature under normal atmospheric pressure, and we have described the optical properties of these arrays. The proposed method is energy efficient and requires no complex experimental procedures or equipment, no surfactant, and no template. Interestingly, 5 nm diameter ZnO nanorod arrays could also be synthesized at 0 °C. We speculate that the oxygen-rich conditions and low reaction temperatures are key factors for the formation of the 5 nm ZnO nanorods. The present findings should be applicable to the synthesis of other oxide nanostructure arrays with small diameters.Acknowledgements
This work was supported by grants from the second phase BK21 program of the Ministry of Education of Korea and the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (Grant No. 2010-0000797).References
- Ü. Özgür, Ya. I. Alivov, C. Liu, A. Teke, M. A. Reshchikov, S. Doğan, V. Avrutin, S.-J. Cho and H. Morkoç, J. Appl. Phys., 2005, 98, 041301 CrossRef.
- Y. Fang, Q. Pang, X. Wen, J. Wang and S. Yang, Small, 2006, 2, 612 CrossRef CAS.
- M. Yin, Y. Gu, I. L. Kuskovsky, T. Andelman, Y. Zhu, G. F. Neumark and S. O'Brien, J. Am. Chem. Soc., 2004, 126, 6206 CrossRef CAS.
- Y. Li, L. Guo, H. Xu, L. Ding, C. Yang, J. Wang, W. Ge, S. Yang and Z. Wu, J. Appl. Phys., 2006, 99, 114302 CrossRef.
- Y.-A. Chung, Y.-C. Chang, M.-Y. Lu, C.-Y. Wang and L.-J. Chen, J. Electrochem. Soc., 2009, 156, F75 CrossRef CAS.
- M. S. Arnold, P. Avouris, Z. W. Pan and Z. L. Wang, J. Phys. Chem. B, 2003, 107, 659 CrossRef CAS.
- G. S. T. Rao and D. T. Rao, Sens. Actuators, B, 1999, 55, 166 CrossRef.
- (a) C. J. Lee, T. J. Lee, S. C. Lyu, Y. Zhang, H. Ruh and H. J. Lee, Appl. Phys. Lett., 2002, 81, 3648 CrossRef CAS; (b) H. Zeng, X. Xu, Y. Bando, U. K. Gautam, T. Zhai, X. Fang, B. Liu and D. Golberg, Adv. Funct. Mater., 2009, 19, 3165 CrossRef CAS.
- K. Tominaga, N. Umezu, I. Mori, T. Ushiro, T. Moriga and I. Nakabayashi, Thin Solid Films, 1998, 334, 35 CrossRef CAS.
- M. H. Huang, S. Mao, H. Feick, H. Yan, Y. Wu, H. Kind, E. Weber, R. Russo and P. Yang, Science, 2001, 292, 1897 CrossRef CAS.
- Z. R. Tian, J. A. Voigt, J. Liu, B. Mckenzie, M. J. Mcdermott, M. A. Rodriguez, H. Konishi and H. F. Xu, Nat. Mater., 2003, 2, 821 CrossRef CAS.
- J. Yang, G. Liu, J. Lu, Y. Qiu and S. Yang, Appl. Phys. Lett., 2007, 90, 103109 CrossRef.
- B. Liu and H. C. Zeng, Langmuir, 2004, 20, 4196 CrossRef CAS.
- Z. R. Tian, J. A. Voigt, J. Liu, B. Mckenzie and M. J. Mcdermott, J. Am. Chem. Soc., 2002, 124, 12954 CrossRef CAS.
- Y. Liu, J. Mao, P. Jiang, Z. Xu, H. Yuan and D. Xiao, CrystEngComm, 2009, 11, 2285 RSC.
- P. Jiang, J.-J. Zhou, H.-F. Fang, C.-Y. Wang, Z. L. Wang and S.-S. Xie, Adv. Funct. Mater., 2007, 17, 1303 CrossRef CAS.
- L. N. Dem'yanets, D. V. Kostomarov and I. P. Kuz'mina, Inorg. Mater., 2002, 38, 124 CrossRef CAS.
- R. A. Lauorse and E. D. Kolb, Am. Mineral., 1963, 48, 642.
- Y. Gu, I. L. Kuskovsky, M. Yin, S. O'Brien and G. F. Neumark, Appl. Phys. Lett., 2004, 85, 3833 CrossRef CAS.
- X. D. Wang, Y. Ding, C. J. Summers and Z. L. Wang, J. Phys. Chem. B, 2004, 108, 8773 CrossRef CAS.
- R. T. Senger and K. K. Bajaj, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 68, 045313 CrossRef.
Footnote |
| † Electronic Supplementary Information (ESI) available: EDX pattern of the ZnO nanorods synthesized from the reaction with the aqueous 0.2 M Na2O2 solution at room temperature for 2 h (Figure S1). SEM images of the surface of the substrates after the reaction with the aqueous 0.2 M Na2O2 solution at room temperature for different reaction times (Figure S2). SEM images of the surface of the substrates after the reaction with the aqueous NaOH solution (pH 13.9) at room temperature (Figure S3). SEM image of the ZnO nanorod arrays synthesized from the reaction with the aqueous ammonia solution at 90 °C for 2 h (Figure S4). See DOI: 10.1039/c0nr00278j/ |
| This journal is © The Royal Society of Chemistry 2010 |
