Ordered mesoporous carbon/α-alumina nanosheet composites†
Joanna
Górka
a,
Mietek
Jaroniec
*a and
Wojciech L.
Suchanek
b
aDepartment of Chemistry, Kent State University, Kent, Ohio 44242, USA. E-mail: jaroniec@kent.edu; Fax: (+1)-330-672-3816; Tel: (+1)-330-672-3790
bSawyer Technical Materials, LLC, 35400 Lakeland Boulevard, Eastlake, Ohio 44095, USA
First published on 9th September 2010
Abstract
Novel α-alumina crystalline nanosheets are used for the preparation of alumina–carbon composites, in which the latter component is phenolic resin-based ordered mesoporous carbon. A unique feature of these composites is perpendicular orientation of ordered mesopores of the carbon to the (001) facets of nonporous α-alumina nanosheets accompanied by significant enlargement of these mesopores in comparison to those present in the bulk carbon.
Introduction
Recently reported soft-templating synthesis of ordered mesoporous carbons (OMCs)1 has a great impact on the development of wide range of carbon-based nanomaterials. The use of triblock copolymers as soft-templates and inexpensive phenolic resins, which can directly interact with hydrophilic domains of block copolymers to form mesostructure in a single step, greatly simplifies the synthesis of OMCs in comparison to the commonly used nanocasting method.2 Moreover, it appeared to be a very efficient method to produce nanocomposites, where desired inorganic nanoparticles can be either generated in situ from the suitable precursors added to the synthesis gel3a–d or directly introduced into the synthesis mixture.3e,f It was shown that the self-assembly process does not suffer a major disruption even in the case of large loadings of introduced inorganic nanoparticles into reaction mixture.3d–f Also, the soft-templating strategy is well suited for the preparation of OMCs in the confined geometries such as channels of anodic alumina,4a,b which resulted in the circular or helical mesopores oriented along the length of alumina channels. Interestingly, this orientation was not found for OMCs formed on non-porous alumina supports.4cIn recent years, a new class of nanomaterials called nanosheets attracted a lot of attention due to their possible applications in catalysis, energy and gas storage, sensors and electronics design.5 Among several methods proposed to obtain these layer-type materials, the most common ones are those based on either physical (thermal evaporation and physical exfoliation) or soft chemical processing (hydrothermal/solvothermal, surfactant- and molecular assembly-assisted templating).6 Unfortunately, some of the aforementioned methods afford layered structures with very low yield, which greatly limits their applications. An exception in this field is the recent work by Yang et al.,6d showing the synthesis of graphene–silica structures, which can be easily fabricated in larger quantities because there are feasible recipes for the preparation of both graphene and silica. This example shows that nanosheets, especially inorganic ones, are attractive components for the fabrication of nanostructured composites because of their 2-dimensional morphology, high crystallinity, well-defined composition, and importantly, the presence of large-area crystallographic facets.
Novel α-alumina nanosheets were recently developed by Suchanek and Garcés7a using the hydrothermal technique that is environmentally friendly and allows for high-yield α-alumina crystallization at ∼400 °C under moderate pressures.7b High-temperature stability (>1000 °C) of these α-alumina nanosheets in addition to unique and easily tailored chemical and physical properties makes them attractive for a variety of catalytic, abrasive, and structural applications.7a
Here we report for the first time the use of the α-alumina nanosheets for the preparation of alumina–carbon composites, in which the latter component is a phenolic resin-based carbon with hexagonally ordered mesopores.1e As illustrated in Fig. 1, a unique feature of these composites is the surface-induced perpendicular orientation of ordered mesopores of this carbon to the (001) facets of nonporous α-alumina nanosheets accompanied by enlargement of the diameter of these pores.
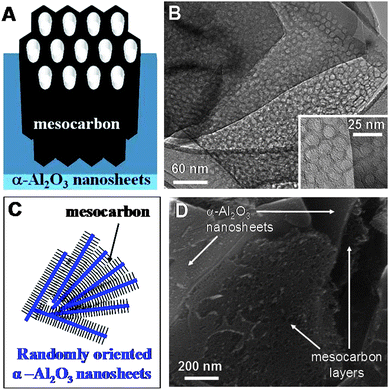 | ||
| Fig. 1 Simple model of ordered mesoporous carbon–α-alumina nanosheet composites supported by HRTEM and SEM images. Carbon layers with mesopores oriented perpendicularly to the (001) facets of α-alumina nanosheets (panels A and B). Higher magnification of the mesopores is shown in the lower inset of B. Several nanosheets with layers of mesoporous carbon between them (panels C and D). The exposed layers of carbon in D reveal the presence of mesopores oriented perpendicularly to the underlying nanosheets. | ||
Experimental section
Sample preparation
The α-alumina nanosheets studied were hydrothermally synthesized at Sawyer Technical Materials, LLC (Eastlake, OH) following the procedure reported elsewhere7 (for details see ESI†). The 20–35 nm thick nonporous nanosheets with exposed (001) facets were identified as α-alumina (corundum, α-Al2O3) with high aspect ratio of 10–200. Mesoporous carbon/α-alumina composites were prepared according to a slightly modified recipe of Wang et al.1e In a typical synthesis, 0.625 g of resorcinol (from Acros Organics) and 0.625 g of poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) triblock copolymer (Pluronic F127 from BASF Corp.) were dissolved in 4.62 g of ethanol–water (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 wt ratio) solution and stirred vigorously at room temperature. After complete copolymer dissolution, alumina nanosheets were added to the reaction mixture, followed by short stirring and the addition of hydrochloric acid (Fischer) as a catalyst. The resulting solution was stirred for additional 30 min. Subsequently, 0.625 mL of 37% formaldehyde (from Acros Organics) was added to the synthesis mixture. The resulting mixture turned to be cloudy after ∼1 h stirring; finally, it separated into two layers. The polymer-rich bottom layer obtained after separation was transferred to a Petri dish and treated at 100 °C for 24 h. Carbonization of the resulting film was performed in the tube furnace under nitrogen flow using a heating rate of 2 °C min−1 up to 180 °C, keeping the sample at this temperature for 5 h, resuming heating with 2 °C min−1 up to 400 °C and with 5 °C min−1 up to 850 °C, and finally keeping the sample at 850 °C for 2 h. For comparison, the sample containing 30% of alumina nanosheets was carbonized at 1100 °C and denoted as C–30*ANS. The final samples were labelled C–xANS, where x indicates the weight percentage of alumina nanosheets (ANS) in the carbon samples. For the purpose of comparison, one sample of pure carbon (C) was prepared using the same procedure but without addition of α-Al2O3 nanosheets.
7 wt ratio) solution and stirred vigorously at room temperature. After complete copolymer dissolution, alumina nanosheets were added to the reaction mixture, followed by short stirring and the addition of hydrochloric acid (Fischer) as a catalyst. The resulting solution was stirred for additional 30 min. Subsequently, 0.625 mL of 37% formaldehyde (from Acros Organics) was added to the synthesis mixture. The resulting mixture turned to be cloudy after ∼1 h stirring; finally, it separated into two layers. The polymer-rich bottom layer obtained after separation was transferred to a Petri dish and treated at 100 °C for 24 h. Carbonization of the resulting film was performed in the tube furnace under nitrogen flow using a heating rate of 2 °C min−1 up to 180 °C, keeping the sample at this temperature for 5 h, resuming heating with 2 °C min−1 up to 400 °C and with 5 °C min−1 up to 850 °C, and finally keeping the sample at 850 °C for 2 h. For comparison, the sample containing 30% of alumina nanosheets was carbonized at 1100 °C and denoted as C–30*ANS. The final samples were labelled C–xANS, where x indicates the weight percentage of alumina nanosheets (ANS) in the carbon samples. For the purpose of comparison, one sample of pure carbon (C) was prepared using the same procedure but without addition of α-Al2O3 nanosheets.
Characterization
Nitrogen adsorption isotherms were measured at −196 °C on ASAP 2010 volumetric analyzer (Micromeritics, Norcross, GA, USA). Prior to the adsorption measurements, all samples were outgassed at 200 °C for at least 2 h.Wide angle X-ray diffraction measurements were performed using a PANalytical X'Pert PRO MPD X-ray diffraction system using Cu Kα radiation (40 kV, 40 mA). All patterns were recorded using 0.02° step size and 4 s per step in the range of 10° ≤ 2θ ≤ 80°. Small angle powder XRD measurements were conducted in the range of 0.4° ≤ 2θ ≤ 3.5° using 0.01° step size and 20 s step time.
Thermogravimetric analysis was performed using a TA Instrument Hi-Res TGA 2950 thermogravimetric analyzer from 30 to 800 °C under air flow with a heating rate of 10 °C min−1.
The morphology of the composites studied was examined by scanning electron microscopy (SEM) at 5 kV accelerating voltage using dual beam FIB (focused ion beam) system of the type xT Nova Nanolab 200 (FEI Company, Hillsboro, Oregon). Prior to the SEM analysis, the samples were ground into fine powder, attached to aluminium holders using a conductive carbon tape, and subsequently sputtered with a thin layer of palladium.
High-resolution transmission electron microscopy (HRTEM) was employed to obtain images of the mesoporous carbon–α-alumina nanosheet samples using field-emission gun energy-filtering high-resolution analytical scanning transmission electron microscope Tecnai F30 (FEI Company, Hillsboro, Oregon) with the acceleration voltage of 300 kV. Prior to the HRTEM analysis, fine-ground samples were dispersed by 5 min ultrasonication in alcohol and then transferred onto Cu grids, and dried under IR heat lamp.
The Brunauer–Emmett–Teller (BET) specific surface area,10SBET, was calculated from nitrogen adsorption isotherms in the range of relative pressures from 0.05 to 0.2 using a cross-sectional area of 0.162 nm2 per nitrogen molecule. The single-point pore volume,11Vt, was estimated from the volume adsorbed at a relative pressure of ∼0.99. The pore size distributions were calculated from nitrogen adsorption isotherms at −196 °C using the improved KJS method9 for cylindrical mesopores with diameters up to 19 nm.
Results and discussion
The wt% of α-alumina nanosheets in the composite was estimated by thermogravimetric analysis (Fig. S1 in ESI†). The sample marked with asterisk was carbonized at 1100 °C in contrast to other samples carbonized at 850 °C. As can be seen from the wide angle XRD patterns (Fig. 2) α-alumina present in the alumina–carbon composite retains its phase composition and crystallinity after the carbonization process, while a characteristic peak at 20–25° for carbon becomes clearly visible. | ||
| Fig. 2 Wide-angle (A) and small angle (B) XRD patterns for α-alumina nanosheets (ANS) and carbon–α-alumina composite (C–30ANS). | ||
Due to rather complex nature of agglomeration and orientations of the nanosheets in the composites, a range of microstructures was obtained, as shown in Fig. 1 and 3. The basic unit of the composite is a single α-alumina nanosheet covered with a layer of mesoporous carbon. In this basic unit, the mesopores are perpendicularly aligned to the surface of the α-alumina nanosheets, as shown schematically in Fig. 1A and confirmed by detailed HRTEM analysis (Fig. 1B and S2 in ESI†). This surface-induced perpendicular orientation of mesopores to α-alumina nanosheets is the effect of interactions between distinct (001) facets of α-alumina and phenolic resin mesostructure. These basic units can form more complex structures when several nanosheets are close to each other (Fig. 1C and D). The α-alumina nanosheets are rarely aligned in a parallel fashion; typically they are oriented to each other under various angles. The SEM images in Fig. 3 and S3 (ESI†) show the bulk of the synthesized α-alumina/carbon composites, which consists of rather homogenously distributed α-alumina nanosheets in the carbon mesostructure. The α-alumina nanosheets form large agglomerates, in which the nanosheets are randomly oriented and covered with mesoporous carbon (Fig. 3). The relatively thin layers of carbons are formed on individual nanosheets, but in the case of composites with higher carbon/alumina ratio the separate domains of bulk mesoporous carbon can be formed (Fig. 3 and S3 in ESI†). Under conditions used in this study the bulk carbon shows hexagonally ordered mesopores, much smaller than those formed on the nanosheets, as illustrated in Fig. S4 (ESI†).
 | ||
| Fig. 3 SEM image of mesoporous carbon/α-alumina composite, C–30ANS, which consists of large agglomerates of randomly oriented nanosheets that are covered by mesoporous carbon; at higher carbon/alumina ratio the bulk mesoporous carbon can be formed in spaces between these sheets too. | ||
In our previous work3e related to the incorporation of 50 nm amorphous alumina nanoparticles into the phenolic resin-based carbon mesostructure a significant reduction in the pore volume was reported with increasing loading of alumina nanoparticles. This led to the conclusion that the mesopore formation via self-assembly process can be affected by differences in the surface properties of the nanoparticles used. In this work, the use of the single-crystal α-alumina nanosheets with distinct (001) facets, which are much more energetically homogenous, resulted in weaker interactions between these facets and phenolic resin interface. Consequently, a perpendicular orientation of phenolic resin-based carbon mesochannels on the α-alumina (001) facets was favored instead of parallel alignment, which resulted in unique structure of the α-alumina–OMC composites. This finding is not surprising in the light of numerous reports showing unique self-organization of block copolymers on various flat surfaces.8 These reports show that the smooth, rigid and neutral surfaces promote the perpendicular orientation to the confining pore walls, which is thermodynamically favored even when the upper surface is removed. Also, the diameters of these channels are often larger than those observed in the bulk phases. The present study shows that the aforementioned mesostructures of block copolymers on the flat surfaces8 can be used to prepare new composites because these mesostructures are not significantly disturbed by incorporation of phenolic resin precursors. The thermosetting nature of phenolic resin permits the removal of block copolymer templates upon thermal treatment in an inert gas (e.g., argon or nitrogen) followed by carbonization process without destroying the ordered mesostructure formed on the α-alumina nanosheets.
Nitrogen adsorption isotherms and the corresponding pore size distributions (PSDs) for the composites studied are presented in Fig. 4, whereas the corresponding adsorption parameters such as the BET specific surface area, the pore volume and pore widths are listed in Table 1. All adsorption isotherms are of type IV according to the IUPAC classification, which is typical for materials with channel-like mesopores. The steep capillary condensation steps at relative pressure of ∼0.6 for C (carbon without alumina), C–20ANS and C–30ANS suggest high uniformity of primary mesopores, which stays in a good agreement with data obtained from small angle XRD and TEM analysis (see Fig. 2 and S4 in ESI†). Importantly, the isotherms for C–20ANS and C–30ANS show some evidence for the presence of larger mesopores as reflected by an increase of adsorption in the relative pressure range of 0.8–0.9. In order to explain this behaviour, two samples (C–66ANS and C–70ANS) with high loading of alumina nanosheets were prepared to eliminate or at least significantly reduce the amount of bulk OMC (not deposited on alumina nanosheets). As can be seen in Fig. 4, the isotherm curves for C–66ANS and C–70ANS show the steps of capillary condensation at higher relative pressures, which confirms the formation of larger mesopores on alumina nanosheets than those present in the bulk carbon. In addition, these larger carbon mesopores are perpendicularly oriented on the (001) facets of α-alumina nanosheets (as shown in Fig. 1 and S2 in ESI† by HRTEM analysis). Thus, a substantial increase in the α-alumina loading resulted in the larger amount of the OMC formed on the (001) facets of α-alumina nanosheets, which is reflected by a significant change in the PSD curves from one mesopore peak with a shoulder to a double mesopore peak (see the right panels in Fig. 4). This suggests that larger polymeric micelles are formed on the (001) facets of α-alumina than those in the bulk aqueous phase. As mentioned above an analogous behaviour was observed in the case of the block copolymer films formed on flat surfaces.8
 | ||
| Fig. 4 Nitrogen adsorption isotherms (left panels) and the corresponding pore size distributions (right panels) for the OMC–alumina nanosheet composites studied (C–30ANS, C–30*ANS, and C–66ANS) and phenolic resin-based OMC (C) (panel A) and for the C–20ANS and C–70ANS composites (panel B). | ||
| Sample | S BET/m2 g−1 | V t/cm3 g−1 | V mi/cm3 g−1 | V me/cm3 g−1 | w/nm |
|---|---|---|---|---|---|
| a Notation: SBET—BET specific surface area; Vt—single-point pore volume; Vmi—volume of fine pores (mainly micropores) obtained by integration of PSD up to 3 nm; Vme—volume of mesopores obtained by integration of PSD from 3 nm to 20 nm; wKJS—mesopore diameter at the maximum of the PSD curve obtained by the improved KJS method.10 | |||||
| C | 813 | 0.70 | 0.08 | 0.50 | 6.5 |
| C–20ANS | 671 | 0.63 | 0.06 | 0.46 | 6.8 |
| C–30ANS | 650 | 0.60 | 0.07 | 0.44 | 6.5 |
| C–30*ANS | 685 | 0.64 | 0.08 | 0.47 | 6.4 |
| C–66ANS | 383 | 0.53 | 0.03 | 0.46 | 8.4/12.8 |
| C–70ANS | 312 | 0.49 | 0.02 | 0.42 | 13.6 |
Also, the data listed in Table 1 show that an increase in the loading of α-alumina nanosheets caused a decrease in the BET surface area and the total pore volume, which can be easily explained by simultaneous reduction of the adsorbing carbon in the composite samples. The BET surface area, pore width, total, mesopore and micropore volumes for the samples with smaller loadings of α-alumina nanosheets were found to be about 700 m2 g−1, 6.5 nm, 0.60 cm3 g−1, 0.45 cm3 g−1 and 0.06 cm3 g−1, respectively, which are typical values for phenolic resin-based carbons; however, the corresponding values for C–66ANS and C–77ANS are smaller. Since α-alumina nanosheets can easily sustain high temperatures,7a one of the samples was carbonized in flowing nitrogen at 1100 °C (C–30*ANS) to prove the exceptional thermal stability of these materials. As can be seen from Fig. 4 and Table 1, thermal treatment did not cause any structure deteriorations, which can be very useful for high temperature applications.
Potential applications of such composites include catalysis, where high surface area of thermally stable alumina and mesoporous carbon is desired.7 Strong faceting of the α-alumina nanosheets is very important here, because it can have a tremendous impact on catalytic selectivity.12 The α-alumina nanosheets can also act as a reinforcement of mesoporous carbon thus increasing the mechanical strength of the material. The oriented mesopores could be used as hard templates for the synthesis of oxide nanofiber/α-alumina nanosheet composites. This work shows the possibility of creating novel composite materials, the structural properties of which can be altered due to interfacial interactions, which in this case resulted in the aforementioned orientation and enlargement of carbon mesopores.
Conclusions
In conclusion, a new type of OMC–alumina composites was successfully prepared by employing single-crystal α-alumina nanosheets in the soft-templating synthesis of hexagonally ordered mesoporous carbons. Such composites feature perpendicular orientation of mesochannels to the surface of highly crystalline α-alumina nanosheets as well as the size of these channels is significantly enlarged, which make them promising materials for catalysis and energy-related applications.This work was supported by National Science Foundation under CHE-0848352 grant. The authors thank BASF Co. for providing the triblock copolymer.
Notes and references
- (a) C. D. Liang and S. Dai, J. Am. Chem. Soc., 2006, 128, 5316 CrossRef CAS; (b) F. Q. Zhang, Y. Meng, D. Gu, Y. Yan, C. Z. Yu, B. Tu and D. Y. Zhao, J. Am. Chem. Soc., 2005, 127, 13508 CrossRef CAS; (c) C. Liang, Z. Li and S. Dai, Angew. Chem., Int. Ed., 2008, 47, 3696 CrossRef CAS; (d) Y. Wan, Y. Shi and D. Y. Zhao, Chem. Mater., 2008, 20, 932 CrossRef CAS; (e) X. Wang, C. Liang and S. Dai, Langmuir, 2008, 24, 7500 CrossRef CAS.
- (a) R. Ryoo, S. H. Joo, M. Kruk and M. Jaroniec, Adv. Mater., 2001, 13, 677 CrossRef CAS; (b) A. Vinu, Top. Catal., 2010, 53, 291 CrossRef CAS; (c) A.-H. Lu and F. Schűth, Adv. Mater., 2006, 18, 1793 CrossRef CAS.
- (a) R. Liu, Y. Ren, Y. Shi, F. Zhang, L. Zhang, B. Tu and D. Y. Zhao, Chem. Mater., 2008, 20, 1140 CrossRef CAS; (b) P. Gao, A. Wang, X. Wang and T. Zhang, Chem. Mater., 2008, 20, 1881 CrossRef CAS; (c) Y. Zhai, Y. Dou, X. Liu, B. Tu and D. Y. Zhao, J. Mater. Chem., 2009, 19, 3292 RSC; (d) J. Yao, L. Li, H. Song, C. Liu and X. Chen, Carbon, 2009, 47, 436 CrossRef CAS; (e) J. Gorka and M. Jaroniec, J. Phys. Chem. C, 2008, 112, 11657 CrossRef CAS; (f) M. Jaroniec, J. Gorka, J. Choma and A. Zawislak, Carbon, 2009, 47, 3034 CrossRef CAS.
- (a) M. Steinhart, C. Liang, G. W. Lynn, U. Gösele and S. Dai, Chem. Mater., 2007, 19, 2383 CrossRef CAS; (b) K. Wang, W. Zhang, R. Phelan, M. A. Morris and J. D. Holmes, J. Am. Chem. Soc., 2007, 129, 13388 CrossRef CAS; (c) K. Kimijima, A. Hayashi and I. Yagi, Chem. Commun., 2008, 5809 RSC.
- (a) A. Takagi, M. Sugisawa, D. Lu, J. N. Kondo, M. Hara, K. Domen and S. Hayashi, J. Am. Chem. Soc., 2003, 125, 5479 CrossRef; (b) D. Li, M. B. Müller, S. G. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3, 101 CrossRef CAS; (c) Y. Zhou, R. Ma, Y. Ebina, K. Takeda and T. Sasaki, Chem. Mater., 2006, 18, 1235 CrossRef CAS; (d) S. Chen, Y. Liu, C. Shao, R. Mu, Y. Lu, J. Zhang, D. Shen and X. Fan, Adv. Mater., 2005, 17, 586 CrossRef CAS.
- (a) T. Sasaki, J. Ceram. Soc. Jpn., 2007, 115, 9 CrossRef CAS; (b) A. Atsushi Takagaki, C. Tagusagawa, S. Hayashi, M. Hara and K. Domen, Energy Environ. Sci., 2010, 3, 82 RSC; (c) M. Choi, K. Na, J. Kim, Y. Sakamoto, O. Terasaki and R. Ryoo, Nature, 2009, 461, 246 CrossRef CAS; (d) S. Yang, X. Feng, L. Wang, K. Tang, J. Maier and K. Müllen, Angew. Chem., Int. Ed., 2010, 49, 4795 CAS.
- (a) W. L. Suchanek and J. M. Garcés, CrystEngComm, 2010 10.1039/b927192a; (b) W. L. Suchanek, J. Am. Ceram. Soc., 2010, 93, 399 CrossRef CAS; (c) W. L. Suchanek and J. Garcés, Patent Application US 2010/0159226 A1 (2010).
- (a) G. T. Pickett and A. C. Balazs, Macromolecules, 1997, 30, 3097 CrossRef CAS; (b) I. A. Zucchi, E. Poliani and M. Perego, Nanotechnology, 2010, 21, 185304 CrossRef CAS; (c) K. W. Guarini, C. T. Black and S. H. I. Yeung, Adv. Mater., 2002, 14, 1290 CrossRef CAS; (d) H. Wang, A. B. Djurixic, M. H. Xiea, W. K. Chanb and O. Kutsay, Thin Solid Films, 2005, 488, 529; (e) U. Jeong, D. Y. Ryu, D. H. Kho, I. K. Kim, J. T. Goldbach, D. H. Kim and T. P. Russell, Adv. Mater., 2004, 16, 533 CrossRef CAS; (f) H. Kitano, S. Akasaka, T. Inoue, F. Chen, M. Takenaka, H. Hasegawa, H. Yoshida and H. Nagano, Langmuir, 2007, 23, 6404 CrossRef CAS.
- M. Jaroniec and L. A. Solovyov, Langmuir, 2006, 22, 6757 CrossRef CAS.
- S. Brunauer, P. H. Emmett and E. Teller, J. Am. Chem. Soc., 1938, 60, 309 CrossRef CAS.
- M. Kruk and M. Jaroniec, Chem. Mater., 2001, 13, 3169 CrossRef CAS.
- I. Lee, F. Delbecq, R. Morales, M. A. Albiter and F. Zaera, Nat. Mater., 2009, 8, 132 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis recipe and figures showing additional TEM and SEM images and the TG profiles for alumina nanosheets and carbon–alumina composites. See DOI: 10.1039/c0nr00482k |
| This journal is © The Royal Society of Chemistry 2010 |
