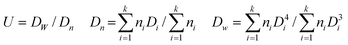Synthesis of P(EGDMA-co-MAA)/P(EGDMA-co-VPy)/titania/polymer tetra-layer microspheres†
Longyu
Li
,
Guangyu
Liu
,
Dianbin
Qin
and
Xinlin
Yang
*
Key Laboratory of Functional Polymer Materials, the Ministry of Education, Institute of Polymer Chemistry, Nankai University, Tianjin, 300071, China. E-mail: xlyang88@nankai.edu.cn; Fax: +86-22-23503510; Tel: +86-22-23502023
First published on 18th February 2010
Abstract
Poly(ethyleneglycol dimethacrylate-co-methacrylic acid)@poly(ethyleneglycol-co-vinylpyridine)/titania/poly(N,N′-methylene bisacrylamide) (P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PMBAAm) and P(EGDMA-co-MAAP)@P(EGDMA-co-VPy)/TiO2/poly(ethyleneglycol dimethacrylate) tetra-layer microspheres were synthesized by distillation precipitation polymerization of N,N′-methylene bisacrylamide (MBAAm) and ethyleneglycol dimethacrylate (EGDMA) in the presence of P(EGDMA-co-MAAP)@P(EGDMA-co-VPy)/TiO2 core-shell particles as the seeds in acetonitrile. The P(EGDMA-co-MAAP)@P(EGDMA-co-VPy)/TiO2 seeds were prepared by a combination of a two-stage distillation precipitation polymerization and the subsequently controlled hydrolysis of titanium tetrabutaoxide (TBOT) in ethanol–acetonitrile mixed solvents. The structures of the resultant polymer and polymer/titania, polymer/titania/polymer multi-layer composite microspheres were confirmed by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM), Fourier-transform infrared spectroscopy (FT-IR) and zeta-potential (ξ) analysis.
Introduction
During the past decade, the fabrication of colloidal materials with definite structural, optical, and surface properties has been an important subject for the researchers both in academic and industrial areas.1–3 Among these materials, inorganic/polymer core-shell composite/hybrid microspheres have attracted increasing attention due to the combination of the thermally stable and robust inorganic components and flexible polymer components with various functional groups within a single material. Furthermore, it has been demonstrated that the structure, size and composition of these composite/hybrid core-shell microspheres can be altered in a controllable way, therefore, their optical, electrical, thermal, mechanical, electro-optical, magnetic, and catalytic properties can be tailored over a wide range. As promising materials, monodisperse core-shell inorganic/polymer microspheres are of importance for potential applications in the areas of drug releasing systems (DDS), coatings, catalysis, diagnostics, and construction of photonic crystals.3–9As a kind of photocatalytic active and photoconductive n-type semiconductor, numerous nano-size titania materials have been synthesized and investigated extensively.10–12 Titania-coated polymer core-shell microspheres and the corresponding hollow titania spheres have been used as photonic band gap materials,13 nano-reactors,14 and catalysts.15,16 Many efforts have been focused on the preparation of titania-coated polymer core-shell composite spheres, which can be generally classified into two categories: coating the polymer particles via the controlled reaction of titania precursors16–18 and decomposition of titania nanoparticles or nanosheets onto polymer microspheres with the aid of the layer-by-layer (LbL) technique.13,19–21 The former method is more attractive for the synthesis of core-shell particles due to its simplicity via a sol–gel procedure. However, the utilization of the sol–gel technique is generally limited by the high activity of the titania precursors and numerous accompanying disadvantages19–21 such as the aggregation of the coated microspheres, the formation of irregular shell-layer and difficulty in controlling the thickness of the titania. Therefore, it is still a challenge to afford a dense, smooth titania coating layer on the surface of polymer microspheres via controlling the hydrolysis rate and the diffusion rate of the titania precursor in the sol–gel system. Tang and co-workers described the synthesis of the core-shell titania-coated polystyrene particles with smooth, homogeneous shells of amorphous ammonia-catalyzed hydrolysis of titanium tetrabutoxide (TBOT) in the ethanol–acetonitrile mixed solvents.22
In our previous work, monodisperse silica/poly(N,N′-methylene bisacrylamide) (SiO2/PMBAAm),23 silica/polydivinylbenzene (SiO2/PDVB) and silica/poly (ethyleneglycol dimethacrylate) (SiO2/PEGDMA)24 core-shell microspheres, ellipsoidal hematite/polymer composite materials,25 tri-layer Au/silica/PMBAAm,26 and ellipsoidal hematite/silica/PDVB27 were successfully prepared by distillation precipitation polymerization in absence of any stabilizer or surfactant. Herein, we described a facile method for the synthesis of P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PMBAAm and P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PEGDMA tetra-layer microspheres via a three-stage reaction, which mainly included the controlled coating of titania-layer (TiO2) onto P(EGDMA-co-MAA)@P(EGDMA-co-VPy) microspheres via controlled hydrolysis of TBOT in acetonitrile–ethanol mixed solvents and the subsequent encapsulation of PMBAAm and PEGDMA outer-layer over polymer/TiO2 seeds to result in poly(ethyleneglycol dimethacrylate-co-methacrylic acid)@poly(ethyleneglycol-co-vinylpyridine)/TiO2/polymer tetra-layer microsphere.
Experimental
Chemicals
4-Vinylpyridine (4-VPy) and methacrylic acid (MAA) were purchased from Acros and Tianjin Chemical Reagent II Co., respectively, which were purified by vacuum distillation. Titanium tetrabutoxide (TBOT), 3-(methacryloxy)propyl trimethoxysilane (MPS), and ethyleneglycol dimethacrylate (EGDMA) were bought from Aldrich and used without any further purification. N,N′-Methylenebisacrylcmide (MBAAm, chemical grade, Tianjin Bodi Chemical Engineering Co.) was recrystallized from acetone. 2,2′-Azobisisobutyronitrile (AIBN) was available from Chemical Factory of Nankai University and recrystallized from methanol. Acetonitrile (analytical grade, Tianjin Chemical Reagents II Co.) was dried over calcium hydride and purified by distillation before use. All the other reagents were analytical grade and utilized without any further purification.Synthesis of monodisperse P(EGDMA-co-MAA)@P(EGDMA-co-VPy) microspheres by a two-stage distillation precipitation polymerization
The preparation of poly(ethyleneglycol dimethacrylate-co-methacrylic acid) (P(EGDMA-co-MAA)) microspheres by first-stage distillation precipitation polymerization was reported in our previous work,28 in which the mol% of the cross-linking monomer was 0.20 and AIBN was used as the initiator. The loading capacity of the accessible carboxylic acid groups on the surface and gel-layer of P(EGDMA-co-MAA) microspheres was 4.00 mmol g−1 as determined by acid–base titration.Poly(ethyleneglycol dimethacrylate-co-methacrylic acid)@poly(ethyleneglycol-co- vinylpyridine) (P(EGDMA-co-MAA)@P(EGDMA-co-VPy)) core-shell microspheres were synthesized by second-stage distillation precipitation polymerization, during which the formation of core-shell microspheres was driven by the efficient hydrogen-bonding interaction between the carboxylic acid groups and the pyridyl group of VPy as described in our previous paper.29 In a typical experiment, 0.20 g of P(EGDMA-co-MAA) microspheres, 0.25 mL (0.26 g, 1.3 mmol) of EGDMA, 0.25 mL (0.25 g, 2.25 mmol) of 4-VPy, and 0.01 g (2 wt% relative to the total comonomers) of AIBN as initiator were dissolved in 80 mL of acetonitrile with ultrasonic irradiation at room temperature in a 100 mL two-necked flask equipped with a fractioning column, a Liebig condenser and a receiver. The flask was submerged in a heating mantle and the reaction mixture was kept under refluxing state for additional 5 min. The solvent was then distilled off the reaction system, and the polymerization was stopped after 40 mL of acetonitrile was distilled from the reaction system within 1.5 h. After the polymerization, the resultant P(EGDMA-co-MAA)@P(EGDMA-co-VPy) microspheres were purified by repeating centrifugation, decantation, and resuspension in acetonitrile with ultrasonic irradiation for three times. The loading capacity of the pyridyl groups on the surface and gel-layer of core-shell polymer microspheres was determined by an acid–base titration similar to the procedure in our previous work.30
Coating P(EGDMA-co-MAA)@P(EGDMA-co-VPy) microspheres with titania shell-layer
The coating reaction was performed in the mixed solvents of ethanol (containing 0.3% water) and acetonitrile by hydrolyzing TBOT at room temperature. For a typical experiment, 0.05 g of P(EGDMA-co-MAA)@P(EGDMA-co-VPy) microspheres with 137 nm of diameter were dispersed in 100 mL of ethanol–acetonitrile (3/1 in v/v) at room temperature. Then, a fresh solution of 2.0 mL TBOT in 20 mL of ethanol–acetonitrile (3/1, v/v) was added to the above suspension containing polymer microspheres with mild stirring. After reacting for 3 h, the P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2 composite particles were cleaned by three cycles of centrifugation and suspension in ethanol with ultrasonic irradiation.Hollow titania spheres by calcination of P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2 composite microspheres
The polymer cores were selectively removed by calcination of the corresponding P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2 composite particles at 600 °C for 4 h in air in a furnace to afford hollow titania spheres. Then hollow titania spheres were cooled to room temperature at a rate of 10 °C min−1.P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PEGDMA tetra-layer microspheres
The MPS-modified P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2 microspheres were afforded by coupling titania-coated P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2 alcosol suspension: excess MPS (1.0 g, 4.0 mmol) was introduced into 51 mL of titania-coated polymer microspheres (containing 0.5 g of particles, 40 mL of ethanol, 10 mL of water and 1.0 mL of aqueous solution of 25% ammonia) in a 100 mL round-bottom flask under stirring. The modification of P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2 particles was achieved by stirring the mixture of titania-coated polymer particles and MPS for 48 h at room temperature. The resultant MPS-modified P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2 particles were purified by three cycles of centrifugation, decantation, and suspension in ethanol with ultrasonic irradiation. The MPS-modified titania-coated polymer particles were dried in a vacuum oven at 50 °C until a constant weight was reached.The MPS-modified P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2 particles were prepared as described above for the further stage of polymerization to afford polymer/titania/polymer tetra-layer hybrid microspheres. A typical procedure for the distillation precipitation polymerization was as follows: 0.10 g of MPS-modified titania-coated polymer seeds were dispersed in 40 mL of acetonitrile as a white suspension in a 50 mL of two-necked flask with the aid of ultrasonic irradiation. Then EGDMA (0.40 mL, 0.39 g, as 1.0 vol% of the reaction system) together with AIBN initiator (0.008 g, 2 wt% corresponding to EGDMA monomer) were dissolved in the above suspension. The two-necked flask attaching with a fractionating column, Liebig condenser and receiver was then submerged in a heating mantle. The reaction mixture was kept under reflux for a further 10 min. The white color deepened during the heating process and the solvent was then distilled off the reaction system. The reaction was stopped after 20 mL of acetonitrile was distilled from the polymerization system within 70 min. After the polymerization, the resultant P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PEGDMA tetra-layer hybrid microspheres were purified by repeating centrifugation, decantation, and resuspension in acetonitrile with ultrasonic-irradiation for three times. The core-shell hybrid particles were finally dried in a vacuum oven at 50 °C until a constant weight was reached.
P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PMBAAm tetra-layer composite microspheres
The distillation precipitation polymerization to afford P(EGDMA-co-MAA)@ P(EGDMA-co-VPy)/TiO2/PMBAAm tetra-layer composites was very similar to that of the typical procedure for preparation of P(EGDMA-co-MAA)@ P(EGDMA-co-VPy)/TiO2/PEGDMA hybrids by varying the monomer from EGDMA to MBAAm (0.40 g) in the presence of unmodified P(EGDMA-co-MAA)@ P(EGDMA-co-VPy)/TiO2 particles as seeds, while the amount of AIBN initiator was maintained at 2 wt% relative to the monomer and the amount of acetonitrile was kept at 40 mL, respectively. The treatment of the multi-layer composite particles was the same as that for the typical procedure for the synthesis of P(EGDMA-co-MAA)@ P(EGDMA-co-VPy)/TiO2/PEGDMA hybrids.The reproducibility of the polymerization was confirmed through several duplicate and triplicate experiments.
Characterization
The morphology and size distribution of P(EGDMA-co-MAA), P(EGDMA-co-MAA)@P(EGDMA-co-VPy), P(EGDMA-co-MAA)@ P(EGDMA-co-VPy)/TiO2, hollow titania sphere, P(EGDMA-co-MAA)@ P(EGDMA-co-VPy)/TiO2/PEGDMA and P(EGDMA-co-MAA)@ P(EGDMA-co-VPy)/TiO2/PMBAAm microspheres were determined by transmission electron microscopy (TEM, Technai G2 20 S-TWIN). All the size and size distribution reflect the averages about 100 particles each, which are calculated according to the following formula:Where, U is the polydispersity index, Dn is the number average diameter, Dw is the weight-average diameter, Di is the diameter of the determined microspheres.
Fourier transform infrared analysis was performed on a Bio-Rad FTS 135 spectrometer with scanning over the range of 400–4000 cm−1.
Zeta potential was checked on a zeta potential analyzer (Zeta Plus, Brookhaven Instrument Co.) by measuring the electrophoretic mobility of the particles with anhydrous ethanol as electrolyte. The concentration of the polyelectrolyte suspension was about 0.001 g mL−1 at room temperature.
X-Ray photoelectron spectroscopy (XPS) analysis was carried out with a PHI 5300 XPS surface analysis system (Physical Electronics, Eden Prairie, MN) using a Mg Kα X-ray source operating at 250 W and 13 kV (hγ = 1253.6 eV). The electronic binding energy of C1s (284.6 eV) was used as the internal standard.
The crystalline structure of the samples was performed on a Japan Rigaku D/max 2550 V X-ray diffractometer using a Cu Kα (0.15465 nm) radiation at 40 kV and 60 mA.
Results and discussion
Scheme 1 illustrated the synthesis of P(EGDMA-co-MAA)@ P(EGDMA-co-VPy)/TiO2/PEGDMA and P(EGDMA-co-MAA)@ P(EGDMA-co-VPy)/TiO2/PMBAAm microspheres via a three-stage reaction, which included the distillation precipitation polymerization to afford the inner P(EGDMA-co-MAA)@P(EGDMA-co-VPy) polymer core, PEGDMA and PMBAAm outer polymer-shell, and the controlled hydrolysis of TBOT in mixed ethanol–acetonitrile solvents to get a titania inorganic mid-layer.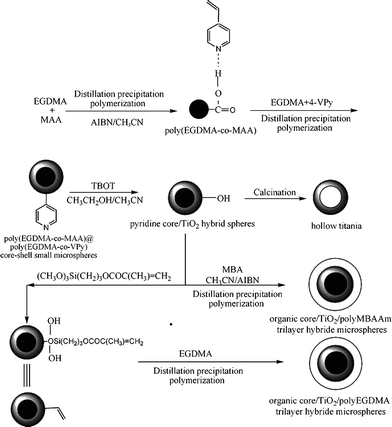 | ||
| Scheme 1 Preparation of P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PMBAAm and P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PEGDMA tetra-layer microspheres. | ||
To get monodisperse polymer microspheres with pyridyl groups on the surface, two-stage distillation precipitation polymerization was used and the typical TEM micrographs of the resultant polymer microspheres are shown in Fig. 1. The results indicated that P(EGDMA-co-MAA), P(EGDMA-co-MAA)@P(EGDMA-co-VPy) microspheres had spherical shape with smooth surfaces in Fig. 1A and 1B, respectively. The average diameter of P(EGDMA-co-MAA) microsphere was 125 nm and a narrow dispersion (U) of 1.007 as summarized in Table 1. The loading capacity of the accessible carboxylic acid groups on the surface of P(EGDMA-co-MAA) microsphere with crosslinking degree of 0.20 was 4.00 mmol g−1, which were used as seeds for the second-stage distillation precipitation polymerization to afford mono-disperse P(EGDMA-co-MAA)@P(EGDMA-co-VPy) microspheres via the efficient hydrogen-bonding interaction between the carboxylic acid groups and the pyridyl groups as illustrated in Scheme 1. The mechanism for the hydrogen-bonding interaction between the pyridyl group and the carboxylic acid group acted as a driving force for the formation of core-shell polymer microspheres31 and the growth of polymer microspheres32 has been studied in detail by our previous works. The size of the core-shell P(EGDMA-co-MAA)@P(EGDMA-co-VPy) microspheres as summarized in Table 1 was 136 nm with a narrow-dispersion (U) of 1.008. This meant that the thickness of P(EGDMA-co-VPy) shell-layer was about 5 nm, which was calculated as half of the difference between the diameter of P(EGDMA-co-MAA) core and that of core-shell P(EGDMA-co-MAA)@P(EGDMA-co-VPy) microspheres. The loading capacity of the accessible pyridyl group on the surfaces and the gel-layer of the core-shell microspheres was 1.91 mmol g−1 as determined by acid–base titration, which permitted the further development of titania coating over the core-shell polymer microspheres.
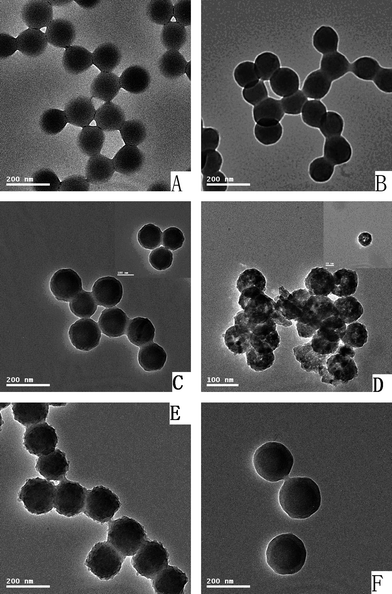 | ||
| Fig. 1 TEM micrographs of particles: (A) P(EGDMA-co-MAA); (B) P(EGDMA-co-MAA)/P(EGDMA-co-VPy); (C) P(EGDMA-co-MAA)/P(EGDMA-co-VPy)/TiO2; (D) Hollow titania spheres; (E) P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PMBAAm tetra-layer composites; (F) P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PEGDMA tetra-layer hybrids. | ||
| Entry | D n /nm | D w /nm | U | TiO2 thickness/nm | Polymer shell thickness/nm |
|---|---|---|---|---|---|
| A | 125 | 136 | 1.007 | ||
| B | 136 | 137 | 1.008 | 5 | |
| C | 146 | 148 | 1.014 | 5 | |
| D | 188 | 190 | 1.011 | 21 | |
| E | 170 | 171 | 1.006 | 12 |
Synthesis of P(EGDMA-co-VPy)/TiO2 composite microspheres
Stable core-shell particles consisting of PSt cores and uniform titania shells were prepared by the hydrolysis of TOBT via the catalysis of ammonium cations in acetonitrile–ethanol mixed solvent to control the hydrolyzing rate and the diffusion rate of the TBOT precursor.22 In our previous work, poly(ethyleneglycol dimethacrylate-co-methacrylic acid)/silica (P(EGDMA-co-MAA)/SiO2) core-shell microspheres were synthesized by coating of an outer-layer of silica onto P(EGDMA-co-MAA) nanoparticles via a modified Stöber process with ammonium as the catalyst.33 Here, P(EGDMA-co-MAA)@P(EGDMA-co-VPy) core particles were uniformly coated with a titania shell in absence of any additional catalyst, during which the pyridyl groups on the surface of polymer cores played an essential role for building the polymer/titania core-shell structure. The efficient interaction between the pyridyl groups on the surface of polymer core and TBOT species acted as a driving force for the uniform encapsulation of the titania shell onto the polymer particles as shown in Scheme 2. The titania shell was strongly bound to the polymer particles with the aid of the coordination effect (Route I) and the hydrogen-bonding interaction (Route II) between the pyridyl groups in the neutral form on the surface of the P(EGDMA-co-MAA)@P(EGDMA-co-VPy) cores and titanium atom, in which the pyridyl group with a lone pair electrons behaved as an electron-donating species and the titanium atom acted as an electron-accepting center. Furthermore, the σ-bonding interaction may occur when the aromatic pyridyl ring adopted a perpendicular orientation to titanium metal surface and π-stacking type interaction might occur in the case of aromatic pyridyl ring parallel to the titania surface, which was much similar to the case for the immobilization of TiO2 particles on different substrates with poly(vinylpyridine) as modifier reported in the literature.34 The essential role of the pyridyl group on the surface of P(EGDMA-co-MAA)@P(EGDMA-co-Py) microsphere for the uniform coating of titania layer onto polymer microspheres was further confirmed by the formation of irregular polymer/titania particles in presence of P(EGDMA-co-MAA) microspheres with carboxylic acid groups as seeds for the hydrolysis of TBOT (TEM micrograph as shown in Fig. S1A in the ESI†), as the pyridyl group was absent in the later case. Furthermore, it was observed that uniform coating of titania onto P(EGDMA-co-MAA)@P(EGDMA-co-VPy) cores was only formed in the case where the water content was less than 0.3% in mixed acetonitrile–ethanol solvent, which indicated that water content in the reaction system was very important for the formation of uniform titania shells with a regular shape during the hydrolysis of TBOT. In such a case, the effect of water content in the mixed acetonitrile–ethanol solvent on the formation of smooth, homogeneous titania coatings may be interpreted by the good matching between the hydrolysis rate of TBOT and the adsorption rate of the corresponding hydrolysate titania onto the surface of P(EGDMA-co-MAA)@P(EGDMA-co-VPy) cores via the efficient interaction between the pyridyl groups and titania species. A typical TEM micrograph of P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2 composites is shown in Fig. 1C with a deep contrast between the P(EGDMA-co-MAA)@P(EGDMA-co-VPy) core and the lighter inorganic titania shell-layer, which indicated that the composite microspheres had spherical shapes and uniform coating of titania shell with good dispersion. The average diameter of P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2 composite microsphere was considerably increased from 136 nm of P(EGDMA-co-MAA)@P(EGDMA-co-VPy) seeds to 146 nm as summarized in Table 1, which implied that titania shell-layer with thickness of 5 nm was encapsulated onto the polymer seeds via the controlled hydrolysis of TBOT in acetonitrile–ethanol mixed solvent.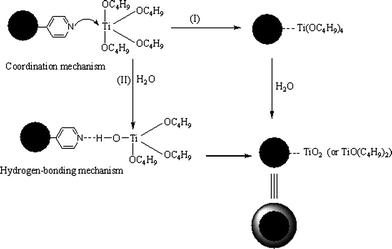 | ||
| Scheme 2 Controlled hydrolysis of TBOT over P(EGDMA-co-MAA)@P(EGDMA-co-VPy) seeds via the efficient interaction between pyridyl groups and titania species. | ||
The preparation of P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2 composite via controlled hydrolysis of TBOT was studied by FT-IR spectra as shown in Fig. 2, in which the FT-IR spectrum in Fig. 2c of polymer/titania core-shell composites had a wide peak in the range of 500–700 cm−1 assigning to the typical stretching vibration of Ti–O–Ti bond of the resultant titania shell-layer. To further prove the core-shell structure of P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2 composite, the surface components of P(EGDMA-co-MAA)@P(EGDMA-co-VPy) seeds and polymer/titania composites were determined by XPS spectra as shown in Fig. 3A and 3B, respectively. The XPS spectrum of P(EGDMA-co-MAA)@P(EGDMA-co-VPy) seeds in Fig. 3A had the strong peaks at 531.4, 398.6, 288.0 eV, which were ascribed to the binding energy of O1s, N1s and C1s, respectively. The XPS spectrum of P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2 composite in Fig. 3B behaved strong peaks at 459 and 464.9 eV assigning to the electronic binding energy of Ti 2p3/2 and Ti 2p1/2, respectively, which were consistent with the results in the literature.35 On the other hand, the peak at 288.0 eV for the electronic binding energy of C1s was significantly decreased with the almost disappearance of the peak at 398.6 eV for binding energy of N1s corresponding to the pyridyl group.
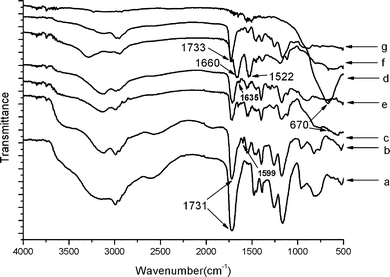 | ||
| Fig. 2 FT-IR spectra: (a) P(EGDMA-co-MAA); (b) P(EGDMA-co-MAA)/P(EGDMA-co-VPy); (c) P(EGDMA-co-MAA)/P(EGDMA-co-VPy)/TiO2; (d) hollow titania spheres; (e) P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PMBAAm tetra-layer composites; (f) MPS-modified P(EGDMA-co-MAA)/P(EGDMA-co-VPy)/TiO2; (g) P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PEGDMA tetra-layer hybrids. | ||
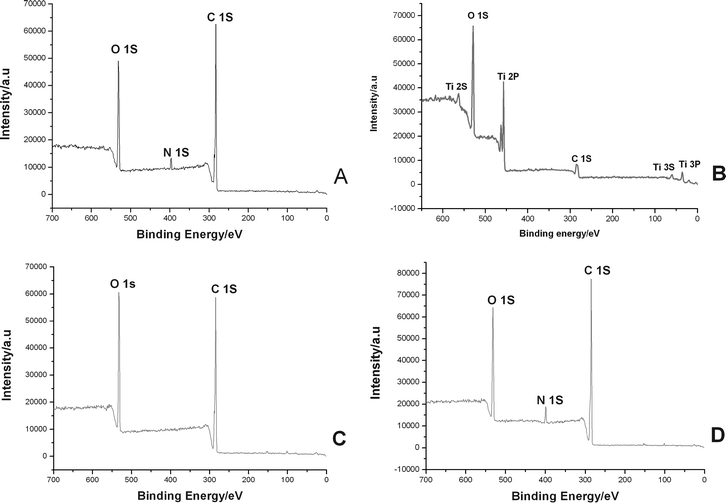 | ||
| Fig. 3 XPS spectra: (a) P(EGDMA-co-MAA)/P(EGDMA-co-VPy); (b) P(EGDMA-co-MAA)/P(EGDMA-co-VPy)/TiO2; (c) P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PMBAAm tetra-layer composites; (d) P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PEGDMA tetra-layer hybrids. | ||
In addition, the zeta-potential of the P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2 composite was −85.0 mV, which implied the surface charge of polymer/titania shell with full coverage of the polymer seeds. This may be originated from the hydroxyl groups of titania component on the surface of polymer/titania composite microspheres via the controlled hydrolysis of TBOT in acetonitrile–ethanol mixed solvent to generate negative charges.
All these results demonstrated that the titania shell-layer with thickness of 5 nm was successfully encapsulated over the P(EGDMA-co-MAA)@P(EGDMA-co-VPy) seeds via the controlled hydrolysis of TBOT in mixed acetonitrile–ethanol solvent in absence of any catalyst with the efficient interaction between the pyridyl groups on the surface of P(EGDMA-co-MAA)@P(EGDMA-co-VPy) seeds and titania species.
Finally, the P(EGDMA-co-MAA)@P(EGDMA-co-VPy) cores were selectively removed by calcination of the P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2 core-shell composite under elevated temperature, which was confirmed by the FT-IR spectrum in Fig. 2d. The results show that the FT-IR spectrum in Fig. 2d of hollow titania sphere had only strong wide peak in the range of 500–700 cm−1 assigned to the characteristic stretching vibration of the Ti–O–Ti bond, while the peak at 1731 cm−1 of the carbonyl group of PEGDMA component disappeared. The morphology of the resultant hollow titania spheres was characterized by TEM with a typical micrograph in Fig. 1D, which indicated that the uncrushed particles retained their spherical shape and a convincing hollow sphere-structure with the presence of a circular-ring of non-segmented particles and a cavity in the interior. The XRD patterns of the core-shell polymer/titania composites and the hollow titania spheres after calcination at 600 °C are displayed in Fig. 4. The XRD pattern in Fig. 4b demonstrates that the hollow titania spheres were transformed from amorphous state as shown in Fig. 4a for polymer/titania core-shell composites to anatase phase with the presence of the diffraction peaks at 2θ of 25.3, 37.9, 48.15, 54.05 and 55.28°, respectively, which were assigned as (101), (004), (200), (105), and (211) reflection planes of the body-centered tetragonal phase of TiO2. This result further proved the selective removal of polymer cores from polymer/titania core-shell composites via calcination to afford hollow titania spheres.
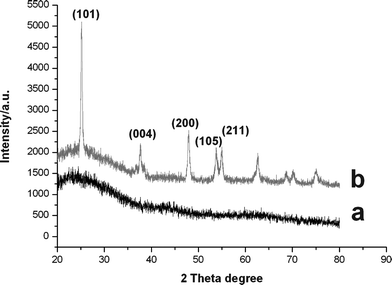 | ||
| Fig. 4 XRD patterns of titania particles: (a) P(EGDMA-co-MAA)/P(EGDMA-co-VPy)/TiO2; (b) hollow titania spheres after calcination. | ||
P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PMBAAm and P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PEGDMA tetra-layer microspheres
In our previous work, monodisperse silica/PMBAAm composites,23 silica/PEGDMA and silica/PDVB core-shell hybrids24 were prepared by distillation precipitation polymerization in the presence of silica and MPS-modified silica particles as seeds, respectively. Herein, P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PMBAAm tetra-layer composites were prepared by distillation precipitation polymerization of N,N′-methylenebisacryl amide (MBAAm) in the presence of P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2 particles as seeds. The PMBAAm was encapsulated over polymer/titania seeds as shown by a typical TEM micrograph in Fig. 1E, which indicated that the tetra-layer composites had a spherical shape with a cauliflower-like surface. This may be due to the high reactivity of MBAAm monomer, which was very similar to the results of distillation precipitation polymerization of MBAAm in our previous work.36 The FT-IR spectrum of the resultant P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PMBAAm tetra-layer composites in Fig. 2f had strong peaks at 1660 and 1522 cm−1 assigned to the stretching vibration of carbonyl groups and bending vibration of N–H bond of PMBAAm outer shell-layer. The average diameter of the resultant tetra-layer composite was enhanced considerably from 148 nm of P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2 tri-layer composites to 171 nm. In other words, PMBAAm shell with thickness of 12 nm was successfully incorporated onto the surface of polymer/titania seeds by the distillation precipitation polymerization of MBAAm in acetonitrile with the aid of the strong hydrogen-bonding interaction between the hydroxyl groups on the surface of polymer/titania seeds and the amide groups of PMBAAm species. The mechanisms of the hydrogen-bonding interaction for the growth of monodisperse poly(methacrylic acid) (PMAA) microspheres,32 construction of SiO2/PMBAAm core-shell particles23 and ellipsoidal hematite/P(MBAAm-co-MAA) core-shell composites25 were investigated in detail by our group. The absence of any secondary-initiated PMBAAm particles in Fig. 1E after the distillation precipitation polymerization implied that the capture ability of P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2 seeds via the hydrogen-bonding interaction was strong enough for the construction of P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PMBAAm tetra-layer composites.Furthermore, the TEM micrograph of MPS-modified P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2 particles as shown in Fig. S1B of the ESI† indicated that these modified particles had a spherical shape and smooth surface with an average diameter of 146 nm and monodispersity index (U) of 1.014, which implied that only a very thin layer of silica-layer was coated onto polymer/titania microspheres to introduce the reactive vinyl group on the surface of the particles. Anyhow, the modification of polymer/titania particles via further hydrolysis of MPS via the self-condensation reaction between surface hydroxyl groups of polymer/titania particles and those of silica component was confirmed by the FT-IR spectrum in Fig. 2e, which displayed a peak at 1635 cm−1 corresponding to the characteristic stretching vibration of the vinyl groups. These reactive vinyl groups permitted the growth of a PEGDMA layer over the MPS-modified P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2 particles by the radical capture of the newly formed oligomers and monomers during the further-stage distillation precipitation polymerization to result in P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PEGDMA tetra-layer hybrid particles. The TEM micrograph of the resultant tetra-layer hybrids is shown in Fig. 1F, demonstrating that the final hybrid microspheres had spherical shapes with smooth surfaces without any secondary initiated particles. This result indicated that the reactive vinyl groups on the surface of MPS-modified polymer/titania captured all the newly formed PEGDMA species to afford tetra-layer hybrid microspheres, which was consistent with the results according to the essential roles of the vinyl groups on the polymer microspheres for the growth of monodisperse polydivinylbenzene (PDVB)37 and PEGDMA microspheres38 in our previous work. The diameter of the resultant tetra-layer hybrid microspheres was 170 nm with a monodispersion (U) of 1.006, which implied that PEGDMA with 12 nm of thickness was encapsulated onto polymer/titania seeds by distillation precipitation polymerization. The successful encapsulation of PEGDMA to result in P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PEGDMA tetra-layer hybrids was confirmed further by the FT-IR spectrum in Fig. 2g with the presence of a strong peak at 1733 cm−1 corresponding to the typical stretching vibration of the carbonyl unit in ester group of PEGDMA component.
To further prove the tetra-layer structure of P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PEGDMA and P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PMBAAm, the surface components of these microspheres were determined by XPS spectra as illustrated in Fig. 3C and 3D, respectively. The XPS spectrum in Fig. 3C of P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PEGDMA tetra-layer hybrids only had strong peaks at 531.4 and 288.0 eV assigned to the electronic binding energy of O1s and C1s from the PEGDMA outer shell-layer. For the XPS spectrum of P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PMBAAm tetra-layer composites in Fig. 3D, only the peaks at 531.4, 398.6 and 288.0 eV corresponding to the electronic binding energy of O1s, N1s and C1s from PMBAAm outer shell-layer were clearly observed. Furthermore, the binding energy of Ti 2p3/2 and Ti 2p1/2 at 459 and 446.9 eV in Fig. 3C and 3D had almost disappeared for the tetra-layer structure microspheres. All these results further confirmed the successful encapsulation of polymer outer shell-layer onto P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2 seeds by the distillation precipitation polymerization via the capture process of polymer species with the aid of either the hydrogen-bonding interaction or the surface vinyl groups on the seeds to afford the tetra-layer structure microspheres.
From the results of the present work, one can access the same surface properties from polymer/TiO2/polymer tetra-layer particles from only PEGDMA and PMBAAm particles but at the cost of a small amount of the polymer because core is replaced by the titania particle as well as the formation of the monodisperse hollow titania microspheres. Secondly, these core-shell hybrids may offer better mechanical, thermal, catalytic and optical properties as compared to the only PEGDMA,38 PMBAAm36 particles, SiO2/PMBAAm core-shell composites23 and SiO2/PEGDMA core-shell hybrids24 in some specific applications, such as for segmented electrophoretic display panel and heterogeneous catalyst.
Conclusion
P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PMBAAm and P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2/PEGDMA tetra-layer microspheres were prepared by multi-stage reaction process, which included combination of distillation precipitation polymerization for the formation of polymer-layers and the controlled hydrolysis of TBOT in acetonitrile–ethanol mixed solvent. The efficient interaction between the pyridyl groups on the surface of P(EGDMA-co-MAA)@P(EGDMA-co-VPy) and the titania species was essential to afford monodisperse tri-layer P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2 microspheres during the controlled hydrolysis of TBOT. PMBAAm and PEGDMA successfully encapsulated P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/TiO2 seeds, during this process the hydrogen-bonding interaction between the amide group of PMBAAm and the hydroxyl group of the polymer/titania seeds and the capture ability of the reactive vinyl groups via the modification of the polymer/titania seeds with MPS played key roles in the construction of the final monodisperse tetra-layer microspheres with tetra-layer structure.Acknowledgements
This work was supported by the National Science Foundation of China with Project No. 20874049.References
- C. H. M. Hofman-Caris, New J. Chem., 1994, 18, 1087–1096 Search PubMed.
- R. Davies, G. A. Schurr, P. Meenan, R. D. Nelson, H. E. Bergna, C. A. S. Brevett and R. H. Goldbaum, Adv. Mater., 1998, 10, 1264–1270 CrossRef CAS.
- L. M. Liz-Marzan, M. Giersig and P. Mulvaney, Langmuir, 1996, 12, 4329–4335 CrossRef CAS.
- Y. Q. Li, S. Y. Fu, Y. Yang and Y. W. Mai, Chem. Mater., 2008, 20, 2637–2643 CrossRef CAS.
- I. Tissot, C. Novat, F. Lefebvre and E. Bourgeat-Lami, Macromolecules, 2001, 34, 5737–5739 CrossRef CAS.
- Y. Lu, J. McLellan and Y. N. Xia, Langmuir, 2004, 20, 3464–3470 CrossRef CAS.
- S. H. Lim, N. S. Phonthammachai, S. Pramana and T. J. White, Langmuir, 2008, 24, 6226–6231 CrossRef CAS.
- J. Luo, X. H. Wang, J. Li, X. J. Zhao and F. S. Wang, Polymer, 2007, 48, 4368–4374 CrossRef CAS.
- V. N. Manoharan, A. Imhof, J. D. Thorne and D. J. Pine, Adv. Mater., 2001, 13, 447–450 CrossRef CAS.
- M. K. Nazeeruddin, A. Kay, I. Rodicio, R. Humpbry-Baker, E. Miiller, P. Liska, N. Vlachopoulos and M. Gratzel, J. Am. Chem. Soc., 1993, 115, 6382–6390 CrossRef CAS.
- J. N. Clifford, E. Palomares, M. K. Nazeerudlin, R. Thampi, M. Gratzel and J. R. Purrant, J. Am. Chem. Soc., 2004, 126, 5670–5671 CrossRef.
- Z. Q. Shi, Z. Y. Li, S. Wang, Z. X. Guo, C. M. Du, S. X. Xiao, N. Sun, Y. A. Gao, J. N. Yao, D. B. Zhu, E. Q. Gao and S. M. Cai, Chem. Phys. Lett., 2001, 336, 19–23 CrossRef CAS.
- H. Nakamura, M. Ishii, A. Tsukiyase, M. Harada and H. Nakano, Langmuir, 2005, 21, 8918–8922 CrossRef CAS.
- Z. Li and H. C. Zeng, Angew. Chem., Int. Ed., 2005, 44, 4342–4345 CrossRef CAS.
- A. Syoufian, O. H. Satriya and K. Nakashima, Catal. Commun., 2007, 8, 755–759 CrossRef CAS.
- M. Zhang, G. Gao, D. C. Zhao, Z. Y. Li and F. Q. Liu, J. Phys. Chem. B, 2005, 109, 9411–9415 CrossRef CAS.
- A. Imhof, Langmuir, 2001, 17, 3579–3585 CrossRef CAS.
- Z. Z. Yang, Z. W. Niu, Y. F. Lu, Z. B. Hu and C. C. Han, Angew. Chem., Int. Ed., 2003, 42, 1943–1945 CrossRef CAS.
- F. Caruso, X. Y. Shi, R. A. Caruso and A. Susha, Adv. Mater., 2001, 13, 740–744 CrossRef CAS.
- R. A. Caruso, A. Susha and F. Caruso, Chem. Mater., 2001, 13, 400–409 CrossRef CAS.
- L. Wang, T. Sasaki, Y. Ebina, K. Kurashima and M. Watanabe, Chem. Mater., 2002, 14, 4827–4832 CrossRef CAS.
- P. Wang, D. Chen and F. Q. Tang, Langmuir, 2006, 22, 4832–4835 CrossRef CAS.
- G. Y. Liu, X. L. Yang and Y. M. Wang, Polymer, 2007, 48, 4385–4392 CrossRef CAS.
- G. Y. Liu, H. Zhang, X. L. Yang and Y. M. Wang, Polymer, 2007, 48, 5896–5904 CrossRef CAS.
- G. Y. Liu, L. Y. Li and X. L. Yang, Polymer, 2008, 49, 4776–4783 CrossRef CAS.
- G. Y. Liu, H. F. Ji, X. L. Yang and Y. M. Wang, Langmuir, 2008, 24, 1019–1025 CrossRef CAS.
- G. Y. Liu, X. L. Yang and Y. M. Wang, Langmuir, 2008, 24, 5485–5491 CrossRef CAS.
- F. Bai, X. L. Yang, R. Li, B. Huang and W. Q. Huang, Polymer, 2006, 47, 5775–5784 CrossRef CAS.
- J. Y. Wang and X. L. Yang, Colloid Polym. Sci., 2008, 286, 283–291 CrossRef CAS.
- S. N. Li, X. L. Yang and W. Q. Huang, Chin. J. Polym. Sci., 2007, 25, 555–564 CrossRef CAS.
- J. Y. Wang and X. L. Yang, Langmuir, 2008, 24, 3358–3364 CrossRef CAS.
- F. Bai, B. Huang, X. L. Yang and W. Q. Huang, Eur. Polym. J., 2007, 43, 3923–3932 CrossRef CAS.
- H. F. Ji, S. P. Wang and X. L. Yang, Polymer, 2009, 50, 133–140 CrossRef CAS.
- S. Malynych, I. Luzinov and G. Chumanov, J. Phys. Chem. B, 2002, 106, 1280–1285 CrossRef CAS.
- C. Viornery, Y. Chevolot, D. Leonard, B. O. Aronsson, P. Pechy, H. J. Mathieu, P. Descouts and M. Gratzel, Langmuir, 2002, 18, 2582–2589 CrossRef CAS.
- G. Y. Liu, X. L. Yang and Y. M. Wang, Polym. Int., 2007, 56, 905–913 CrossRef CAS.
- F. Bai, X. L. Yang and W. Q. Huang, Macromolecules, 2004, 37, 9746–9752 CrossRef CAS.
- F. Bai, X. L. Yang and W. Q. Huang, Eur. Polym. J., 2006, 42, 2088–2097 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: TEM micrographs of P(EGDMA-co-MAA)/titania composites and P(EGDMA-co-MAA)@P(EGDMA-co-VPy)/titania microspheres. See DOI: 10.1039/b9py00302a |
| This journal is © The Royal Society of Chemistry 2010 |