An easy way to the preparation of multi-miktoarm star block copolymers via sequential double click reactions†
Aydan
Dag
,
Hakan
Durmaz
,
Volkan
Kirmizi
,
Gurkan
Hizal
* and
Umit
Tunca
*
Department of Chemistry, Istanbul Technical University, Maslak, 34469, Istanbul, Turkey
First published on 6th April 2010
Abstract
As a new synthetic route, we here employed sequential double click reactions involving azide–alkyne and Diels–Alder reactions for the preparation of multi-miktoarm star block copolymers by using the arm-first approach.
Until recently multiarm star polymers with a cross-linked core (known as multiarm star polymers) have been prepared mostly by living anionic and less frequently cationic polymerization methods.1 However, the introduction of living radical polymerization (LRP) methods, such as metal mediated living radical polymerization often called atom transfer radical polymerization (ATRP), nitroxide mediated radical polymerization (NMP), and reversible addition fragmentation chain transfer (RAFT) polymerization have enabled the preparation of multiarm star polymers from a wide range of monomers under less stringent reaction conditions than those of ionic polymerizations.1,2
Multiarm star polymers are generally prepared by the “arm-first” and the “core-first” approaches using LRP methods. In the arm-first approach, a divinyl cross-linker (e.g. divinylbenzene (DVB)) and a linear macroinitiator (or macromonomer together with a small molecule initiator) with both an appropriate chain-end initiating site for LRP and a functional end-group (F) initially generate pendant vinyl groups during the polymerization of cross-linker from the linear chain. The highly cross-linked core is formed via intermolecular reactions between the chain-end radicals and the pendant double bonds. The structure of the resulting star polymer can be denoted as poly(F-M)n–polyX, where polyX represents a cross-linked core of the star polymer, n is the average number of poly(F-M) arms per star molecule and F is a functional end-group at the star periphery.2 Therefore, a second block, poly(M2)m can easily be introduced to the star by using functional groups established for polymer coupling reactions in order to give star block copolymers, poly(M2)m–b–poly(M1)–polyX.
Finally, in both approaches two requirements should be fulfilled to successfully prepare multiarm star block copolymers with different compositions that cannot be attained by using only LRP routes: high chain end functionality at the periphery of the star and highly efficient polymer coupling reactions for the second block formation. The former can be more successfully met by the arm-first approach, due to loss of initiation functionality during star formation under LRP conditions in the core-first method. The latter feature can easily be accomplished by the click reactions primarily involving copper catalyzed azide–alkyne and Diels–Alder reactions, which have already been used in the preparation of macromolecular structures with various topologies, e.g., block, graft, star, miktoarm star and dendrimer via polymer–polymer coupling.3,4
Here, as a new synthetic route, we employed sequential double click reactions involving azide–alkyne and Diels–Alder for the preparation of multi-miktoarm star block copolymers by using the arm-first approach. Multiarm star PS with both alkyne and anthracene moieties at the periphery, (alkyne-PS)n–polyDVB–(PS-anthracene)n, was simply obtained via ATRP of DVB concurrently initiated with linear α-silyl protected alkyne- and α-anthracene-terminated PS macroinitiators. It is followed by sequential azide–alkyne and Diels–Alder click reactions with PtBA–N3 and α-maleimide terminated poly(methyl methacrylate) (PMMA–MI), respectively, resulting in formation of the target multi-miktoarm star block copolymer (PtBA)m–(PS)n–polyDVB–(PS)n–(PMMA)k (Scheme 1).
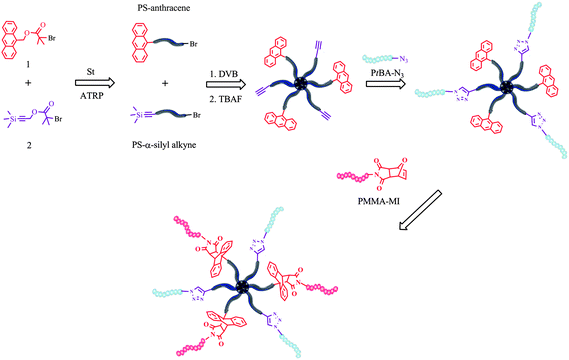 | ||
| Scheme 1 General synthetic strategy to multi-miktoarm star block copolymers. | ||
For the preparation of (alkyne-PS)m–polyDVB–(PS-anthracene)m multiarm star polymer bearing alkyne and anthracene functionalities at the periphery, initially a mixture of linear PS–α-silyl alkyne and PS–anthracene was obtained via one-pot ATRP of St concurrently initiated by using 1 and 2 in the presence of CuBr/PMDETA at 110 °C for 40 min. From the 1H NMR spectrum, the α-anthracene and α-silyl protected alkyne moieties of linear PS were found to be 47 and 53 mol%, respectively according to a ratio of integrated areas of anthracene ArH (δ 8.5–7.4) and –Si(CH3)3 (δ 0.16). Moreover this ratio was consistent with an equimolar feed ratio of 1 to 2 in ATRP of St. GPC number-average molecular weight (Mn,GPC = 5400) and polydispersity index (Mw/Mn = 1.10) were determined by conventional GPC calibrated with PS standards in THF at 30 °C and fit well with those of NMR (Mn,NMR = 6030) and theoretical (Mn,theo = 5100) number-average molecular weight. A mixture of PS–anthracene and PS–α-silyl alkyne was subsequently used as macroinitiator in ATRP of DVB catalyzed by PMDETA/CuBr at 110 °C for the preparation of (α-silyl alkyne-PS)m–polyDVB–(PS-anthracene)m multiarm star polymer bearing alkyne and anthracene at the periphery. The star formation was monitored by GC and GPC (Fig. S1, ESI†) via taking samples at specified time intervals and stopped after 12 h at 94% of DVB conversion. The (α-silyl alkyne-PS)m–polyDVB–(PS-anthracene)m multiarm star polymer was purified by dissolution/precipitation in THF/methanol–ether, respectively. The refractive index increment value (dn/dc = 0.185 mL g−1) of the analyzed multiarm star polymer is calculated experimentally and found to be equal to that of linear PS and after introducing this value to OmniSec software, the absolute molecular weight (Mw) of the multiarm star polymer (α-silyl alkyne-PS)m–polyDVB–(PS-anthracene)m was measured to be 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 using Viscotek triple detection GPC (TD-GPC). Moreover, the number of arms (f) per molecule of the multiarm star polymer was calculated to be 33 using the equation given in the literature.4k Again, 1H NMR analysis of the multiarm star polymer revealed that star polymer contains 46 and 54 mol% of anthracene and silyl-alkyne moieties at the periphery, respectively, using similar NMR assignments to those given above for a mixture of linear PS–α-silyl alkyne and PS–anthracene (Fig. S2, ESI†). Moreover, it should be noted that the number of arms per star molecule containing silyl-alkyne and anthracene moieties is determined to be 17 and 14, respectively.
000 using Viscotek triple detection GPC (TD-GPC). Moreover, the number of arms (f) per molecule of the multiarm star polymer was calculated to be 33 using the equation given in the literature.4k Again, 1H NMR analysis of the multiarm star polymer revealed that star polymer contains 46 and 54 mol% of anthracene and silyl-alkyne moieties at the periphery, respectively, using similar NMR assignments to those given above for a mixture of linear PS–α-silyl alkyne and PS–anthracene (Fig. S2, ESI†). Moreover, it should be noted that the number of arms per star molecule containing silyl-alkyne and anthracene moieties is determined to be 17 and 14, respectively.
Next, silyl groups at the periphery were deprotected by reacting with TBAF at room temperature for 2 h. Deprotection can be followed by monitoring the complete disappearance of –Si(CH3)3 protons at 0.16 ppm using 1H NMR (Fig. S2, ESI†).
After deprotection, (alkyne-PS)m–polyDVB–(PS-anthracene)m multiarm star polymer was reacted with well-defined linear PtBA–N3 (synthetic details given in the experimental part) in the presence of CuBr/PMDETA in DMF at room temperature to obtain (PtBA)n–(PS)m–polyDVB–(PS-anthracene)m multi-miktoarm star block copolymer. A molar ratio of PtBA–N3 to the star molecule was adjusted to be 20 slightly exceeding the number of arms bearing alkyne at the periphery.
Unreacted linear PtBA–N3 was simply removed from the reaction mixture by precipitation in methanol/diethyl ether. NMR analysis showed that PtBA (δ 1.4) precursor had successfully been incorporated into the multiarm star polymer. The dn/dc value for the (PtBA)n–(PS)m–polyDVB–(PS-anthracene)m multi-miktoarm star block copolymer was determined to be 0.146 mL g−1 using an equation, which correlates linearly with weight fractions of PS and PtBA segments (wPS = 0.715 and wPtBA = 0.285) and dn/dc of the linear precursors. For this calculation, DPn of the PS segment in the (PtBA)n–(PS)m–polyDVB–(PS-anthracene)m star polymer and the dn/dc value for the linear PtBA were assumed to be 55 and 0.049 mL g−1, respectively. After introducing dn/dc = 0.146 into the OmniSec of TD-GPC, Mw = 355![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and Mw/Mn = 1.25 of the multi-miktoarm star block copolymer were obtained and found to be consistent with that of Mw,theo = 321
000 and Mw/Mn = 1.25 of the multi-miktoarm star block copolymer were obtained and found to be consistent with that of Mw,theo = 321![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. Moreover, hydrodynamic radius (Rh) and intrinsic viscosity ([η]) values were determined from TD-GPC and are given in Table S1 (ESI†).
000. Moreover, hydrodynamic radius (Rh) and intrinsic viscosity ([η]) values were determined from TD-GPC and are given in Table S1 (ESI†).
Monomodal GPC trace and clear shift with respect to the precursors were observed indicating the successful azide–alkyne click reaction, and thus, the formation of the multi-miktoarm star block copolymer (Fig. 1).
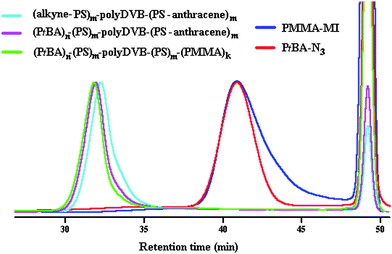 | ||
| Fig. 1 Evolution of GPC traces of star polymers and their corresponding linear precursors in THF at 30 °C. | ||
Next, Diels–Alder click reaction strategy was applied to the preparation of (PtBA)n–(PS)m–polyDVB–(PS)m–(PMMA)k by simply reacting anthracene groups at the periphery of (PtBA)n–(PS)m–polyDVB–(PS-anthracene)m with linear PMMA–MI precursor. The Diels–Alder click reaction was performed in the presence of 1.4-fold molar excess of PMMA–MI per arm of the star molecule bearing anthracene at the periphery. The formation of target multi-miktoarm star block copolymer is followed over time by UV spectroscopy upon the disappearance of the signal of the anthryl group at 366 nm (Fig. S3, ESI†). By using UV data, Diels–Alder click reaction efficiency was found to be over 95%.
The reaction was stopped after 48 h and the remaining solid was purified by a two times dissolution–precipitation cycle in THF–methanol. NMR analysis showed that PMMA block (δ 3.6) was incorporated into the star polymer. The dn/dc value for (PtBA)n–(PS)m–polyDVB–(PS)m–(PMMA)k is calculated to be 0.137 mL g−1 using the equation, which correlates linearly with weight fractions of PS, PtBA and PMMA segments in the star (wPS = 0.63, wPtBA = 0.25, and wPMMA = 0.12), and dn/dc values of linear precursors (Fig. 2).
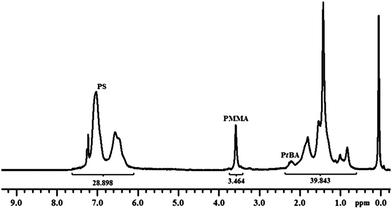 | ||
| Fig. 2 1H NMR spectrum of (PtBA)n–(PS)m–polyDVB–(PS)m–(PMMA)k multi-miktoarm star block copolymer in CDCl3. | ||
For this calculation it is assumed that the DPns of PS and PtBA were 55 and 18, respectively, and the dn/dc value for the linear PMMA was 0.076 mL g−1. Next, after introducing the dn/dc = 0.137 into the OmniSec, Mw,TD-GPC, Rh and [η] for (PtBA)n–(PS)m–polyDVB–(PS)m–(PMMA)k were calculated and are listed in Table S1†. As can be deduced from Table S1 (ESI†), all star polymers have a narrow polydispersity index and a gradual increase in Rh and Mw,TD-GPC values of star polymers confirmed a successful sequential incorporation of blocks into the star. It is also noted that Mw,theo = 385![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 of (PtBA)n–(PS)m–polyDVB–(PS)m–(PMMA)k is fairly consistent with that of Mw,TD-GPC = 407
000 of (PtBA)n–(PS)m–polyDVB–(PS)m–(PMMA)k is fairly consistent with that of Mw,TD-GPC = 407![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. From Fig. 1, it can be seen that a monomodal GPC trace of (PtBA)n–(PS)m–polyDVB–(PS)m–(PMMA)k displayed a slight shift to a lower retention time with respect to that of (PtBA)n–(PS)m–polyDVB–(PS-anthracene)m indicating an incorporation of PMMA segment into the star. Additionally, dn/dc values for all multi-miktoarm star block copolymers were found experimentally and their corresponding Mw,TD-GPC, Rh and [η] values were obtained from the OmniSec of TD-GPC (Table S1, ESI†).
000. From Fig. 1, it can be seen that a monomodal GPC trace of (PtBA)n–(PS)m–polyDVB–(PS)m–(PMMA)k displayed a slight shift to a lower retention time with respect to that of (PtBA)n–(PS)m–polyDVB–(PS-anthracene)m indicating an incorporation of PMMA segment into the star. Additionally, dn/dc values for all multi-miktoarm star block copolymers were found experimentally and their corresponding Mw,TD-GPC, Rh and [η] values were obtained from the OmniSec of TD-GPC (Table S1, ESI†).
In conclusion, we simply achieved multi-miktoarm star block copolymers via exploiting double click reactions. Towards this aim, a mixture of anthracene and alkyne functional small initiators was used in ATRP of St to give a mixture of macroinitiators with end-functionalities as given above. A ratio of end-functionalities of these macroinitiators directly reflects in the star periphery, as macroinitiators were employed in the formation of star polymers via arm-first approach. So the ratio of anthracene and alkyne functionalities at the star periphery can be easily controlled by a suitable choice of the ratio of initiators in the feed of ATRP. In fact, equimolar concentrations of the initiators, 1 and 2, resulted in 17 and 14 arms out of a 31-arm star polymer, (alkyne-PS)m–polyDVB–(PS-anthracene)m, bearing alkyne and anthracene, respectively.
Next, linear PtBA and PMMA blocks can be easily introduced into that 31-arm star polymer, (alkyne-PS)m–polyDVB–(PS-anthracene)m, via sequential double click reactions in order to give well-defined multi-miktoarm star block copolymers, (PtBA)n–(PS)m–polyDVB–(PS-anthracene)m and (PtBA)n–(PS)m–polyDVB–(PS)m–(PMMA)k, with narrow molecular weight distribution and higher molecular weights up to 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. Absolute molecular weight (Mw,TD-GPC), polydispersity index, Rh and [η] for all multiarm star polymers were measured by TD-GPC based on both calculated and experimental dn/dc values and Mw,TD-GPC values were found to be reliable with Mw,theo calculated from a sum of molecular weights of the core and the individual arms.
000. Absolute molecular weight (Mw,TD-GPC), polydispersity index, Rh and [η] for all multiarm star polymers were measured by TD-GPC based on both calculated and experimental dn/dc values and Mw,TD-GPC values were found to be reliable with Mw,theo calculated from a sum of molecular weights of the core and the individual arms.
Notes and references
- (a) N. Hadjichristidis, J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 857 CrossRef CAS; (b) N. Hadjichristidis, M. Pitsikalis, S. Pispas and H. Iatrou, Chem. Rev., 2001, 101, 3747 CrossRef CAS; (c) N. Hadjichristidis, H. Iatrou, M. Pitsikalis and J. Mays, Prog. Polym. Sci., 2006, 31, 1068 CrossRef CAS.
- (a) H. F. Gao and K. Matyjaszewski, J. Am. Chem. Soc., 2007, 129, 11828 CrossRef CAS; (b) H. F. Gao and K. Matyjaszewski, Prog. Polym. Sci., 2009, 34, 317 CrossRef CAS; (c) A. Blencowe, J. F. Tan, T. K. Goh and G. G. Qiao, Polymer, 2009, 50, 5 CrossRef CAS.
- (a) Y. Yagci and M. A. Tasdelen, Prog. Polym. Sci., 2006, 31, 1133 CrossRef CAS; (b) B. S. Sumerlin and A. P. Vogt, Macromolecules, 2010, 43, 1–13 CrossRef CAS; (c) M. Meldal and C. W. Tornoe, Chem. Rev., 2008, 108, 2952 CrossRef CAS; (d) C. R. Becer, R. Hoogenboom and U. S. Schubert, Angew. Chem., Int. Ed., 2009, 48, 4900 CrossRef CAS; (e) M. Ouchi, T. Terashima and M. Sawamoto, Chem. Rev., 2009, 109, 4963 CrossRef CAS; (f) R. K. Iha, K. L. Wooley, A. M. Nystrom, D. J. Burke, M. J. Kade and C. J. Hawker, Chem. Rev., 2009, 109, 5620–5686 CrossRef CAS.
- (a) J. A. Syrett, G. Mantovani, W. R. S. Barton, D. Price and D. M. Haddleton, Polym. Chem., 2010, 1, 102 RSC; (b) Y. Li and T. Michinobu, Polym. Chem., 2010, 1, 72 RSC; (c) A. Dag, H. Durmaz, E. Demir, G. Hizal and U. Tunca, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 6969 CrossRef CAS; (d) H. Durmaz, A. Dag, A. Hizal, G. Hizal and U. Tunca, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 7091 CrossRef CAS; (e) M. Tonga, N. Cengiz, M. M. Kose, T. Dede and A. Sanyal, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 410 CrossRef CAS; (f) E. Gungor, G. Hizal and U. Tunca, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 3409 CrossRef CAS; (g) B. Gacal, H. Akat, D. K. Balta, N. Arsu and Y. Yagci, Macromolecules, 2008, 41, 2401 CrossRef CAS; (h) S. Sinnwell, A. J. Inglis, T. P. Davis, M. H. Stenzel and C. Barner-Kowollik, Chem. Commun., 2008, 2052 RSC; (i) H. Durmaz, A. Dag, D. Gursoy, A. L. Demirel, G. Hizal and U. Tunca, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 1557 CrossRef CAS; (j) S. Sinnwell, M. Lammens, M. H. Stenzel, F. E. Du Prez and C. Barner-Kowollik, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 2207 CrossRef CAS; (k) A. Dag, H. Durmaz, U. Tunca and G. Hizal, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 178 CrossRef CAS; (l) J. T. Wiltshire and G. G. Qiao, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 1485 CrossRef CAS; (m) H. Durmaz, A. Dag, E. Erdogan, A. L. Demirel, G. Hizal and U. Tunca, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 99 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, NMR, UV-Vis spectra, and GPC results. See DOI: 10.1039/c0py00059k |
| This journal is © The Royal Society of Chemistry 2010 |
