Slowing the growth of ice with synthetic macromolecules: beyond antifreeze(glyco) proteins
Matthew I.
Gibson
*
Department of Chemistry, University of Warwick, Coventry, CV4 7AL, UK. E-mail: m.i.gibson@warwick.ac.uk; Fax: +44 (0)247 652 4112; Tel: +44 (0)247 652 4803
First published on 26th May 2010
Abstract
Biological antifreezes are a relatively large and diverse class of proteins (and very recently expanded to include lipopolysaccharides) which are capable of interacting with ice crystals in such a manner as to influence and, under the correct conditions, to prevent their growth. These properties allow for the survival of organisms which are either continuously or sporadically exposed to subzero temperatures which would otherwise lead to cryo-injury/death. These proteins have been found in a range of organisms, including plants, bacteria, insects and fish, and the proteins themselves have a diverse range of chemical structures ranging from the highly conserved antifreeze glycoproteins (AFGPs) to the more diverse antifreeze proteins AFPs. Their unique abilities to non-colligatively decrease the freezing point of aqueous solutions, inhibit ice recrystallisation and induce dynamic ice shaping suggest they will find many applications from cell/tissue/organ cryostorage, frozen food preservatives, texture enhancers or even as cryosurgery adjuvants. However, these applications have been limited by a lack of available material and also underlying questions regarding their mode of activity. The aim of this review article is to highlight the potential of polymeric materials to act as synthetic mimics of antifreeze(glyco) proteins, as well as to summarise the current general challenges in designing compounds capable of mimicking AF(G)Ps. This will cover the basic properties and modes of action of AF(G)Ps along with the methods commonly used to evaluate their activity. This section is essential to specifically define the ‘antifreeze’ terminology in terms of these proteins' unique function and to distinguish them from conventional antifreezes. A detailed evaluation of the processes involved in AF(G)P activity is beyond the scope of this review, but the reader will be pointed towards relevant literature. This will then be placed in the context of modern polymer science, with a focus on the ability of synthetic polymers to display some type of specific antifreeze activity, which will be summarised. Finally, the potential applications of these materials will be highlighted and future avenues for their research and the challenges faced in achieving these goals suggested.
 Matthew I. Gibson | Dr Matthew Gibson was appointed as a Science City Interdisciplinary Research Fellow in the Department of Chemistry at the University of Warwick in December 2009. He also holds an honorary position at the University of Birmingham. He graduated with a 1st class Masters degree from the University of Durham in 2003, and stayed at the same institution to complete his PhD with Professor Neil Cameron in 2007. Following this he moved to EPFL, Switzerland, to work with Professor Harm-Anton Klok as post-doctoral fellow. His current research focuses on the use of macromolecular materials to replace, mimic or interact with biological systems. |
Introduction
Despite average surface temperatures dropping as low as minus 1.9 °C many animal species thrive in the polar/near polar seas. Warm-blooded animals have adapted to this by using additional insulating layers in the form of blubber allowing homeostasis to be maintained. For cold-blooded species this is a more serious issue as their body temperatures can rise and fall with their surroundings and there is the possibility of ice crystals entering their circulation and seeding further ice growth, presenting a significant risk of cell/tissue damage. In response to this environmental stress, polar fish species have significantly higher levels of dissolved solutes in their blood plasma when compared to temperate species, leading to a reduction in the freezing point. Schoolander and DeVries demonstrated that less than 70% of the observed freezing point depression of the blood of marine teleosts could be accounted for on a colligative basis by dissolved salts and sugars.1,2 The remaining 30% depression was found to be due to the presence of a series of circulating glycoproteins. Further examination of other cold-acclimatised polar fish showed that the structure of the glycoproteins was highly conserved, based upon the same regular tripeptide sequence of alanine-alanine-threonine with a complex disaccharide (β-D-galactosyl(1→3)-α-N-acetyl-D-galactosamine) at the threonine residue with some minor sequence variations (Scheme 1). The number of repeat units of this sequence was found to vary from 4 to 50, with the larger mass fractions showing increased antifreeze (defined below) activity. Minor sequence variations are often found and have been discussed previously,3 but AFGPs still represent a remarkable example of convergent evolution.4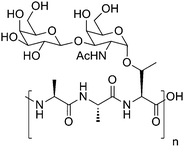 | ||
| Scheme 1 Structure of native AFGP. | ||
A second family of proteins with similar properties known simply as antifreeze proteins (AFPs) are found in marine species but also across several terrestrial species covering insects to plants.5–7 AFPs can be further divided into 4 sub-categories (named AFP I, II, III and IV) which have well-defined structural features, ranging from the single stranded alpha helical, alanine rich type I to the β-sandwich type III AFPs. The structural differences between AFPs will not be considered here and have been the topic of several reviews.4,5,8 Recently several AFPs have been discovered which show antifreeze activity significantly stronger than other AFPs and AFGPs and are referred to as hyperactive AFPs.7
Antifreeze properties and methods of assessment
The term antifreeze is rather generally applied in some literature and it is important to first define what is meant by this term within the context of this review article. Secondly, the use of these terms to define activity is complicated by the variety of methods available to determine them. Considering this we will discuss the key physical methods used for determining antifreeze activity and try to establish trends between the properties, particularly with regard to whether all antifreeze properties are necessarily present in all proteins/mimics. We will use the term antifreeze from this point to imply that one or more of the following physical properties (vide infra) is present. It will not include colligative freezing point depression, such as that caused by addition of salts or ethylene glycol nor will it refer to the nucleation inhibiting behaviour (important during vitrification) which falls outside of this review but will be put into context when appropriate. Ice nucleation proteins, which also play a beneficial role in some freeze-tolerant species, are also excluded here.9,10Terminology
Thermal Hysteresis
The most commonly referred to characteristic of AF(G)Ps is the freezing point depression, but more accurately described using the term Thermal Hysteresis. A freezing point depression occurs upon the addition of any solute to a solvent. This is a colligative (i.e. thermodynamic, as with boiling point elevation and osmotic pressure) effect, whereby the equilibrium freezing/melting point (when the Gibbs free energies of melting and freezing are equal) is lowered proportionally to the concentration of the solute. Solutions containing AF(G)Ps often display a freezing point depression which is greater than that predicted using colligative arguments (i.e. a kinetic effect) and such that the freezing point is lower than melting point. An ice crystal held isothermally within this gap, in an AF(G)P solution will not grow any further or melt, until the temperature is increased/decreased out of this range. This difference is known as the Thermal Hysteresis gap, abbreviated to TH throughout this review. The standard method for determination of this is by observing the growth of single crystals using a nanolitre osmometer.11Dynamic ice shaping/crystal habit modification
This refers to the ability of an antifreeze active compound to influence the morphology of a single ice crystal. Generally, if there is a TH gap the morphology is determined within this range or as close to the freezing point as possible. Temperatures significantly below this level can result in burst growth of the crystals from specific faces. Dynamic ice shaping can be considered to be macroscopic effect as a result of changes to the crystal habit of ice crystals on a molecular level. These will be discussed alongside each other and the appropriate term used depending on what scale the process is being investigated upon. While not a single definition, this is necessary to allow distinction to be made between techniques for analysis and interpretation of results. This can be observed by observing the growth of a single ice crystal (again using a nanolitre osmometer11) hemispherical etching,12 formation of pits during ice growth13 or wide-angle X-ray diffraction.14Fig. 1A–D shows examples of different ice crystal shapes in the presence or absence of AF(G)Ps.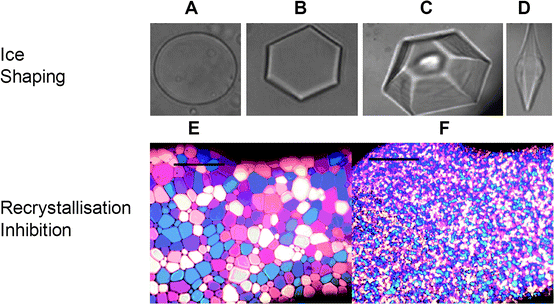 | ||
| Fig. 1 Observation of thermal hysteresis and recrystallisation inhibition activity using optical assays. (A)–(D) show single crystals of ice prepared on a freezing stage of a microscope. (A) Absence of substances which interact with ice; (B) dilute solution of AFP; (C) visualisation of ridges caused by “step-pinning” in the presence of AFP solution; (D) hexagonal bipyramid formed at high concentrations of AFP. (E) and (F) show recrystallisation inhibition assays. Phosphate buffered saline solution prepared using “splat” assay after 30 minutes: (E) no additives; (F) with 5 mg mL−1 poly(vinyl alcohol)2625. Images (A), (B), (C) and (D) are reprinted from TRENDS in Plant Science, Copyright (2004), with permission from Elsevier.21 Images (E) and (F) are reprinted with permission from reference 50 Copyright (2009) American Chemical Society. | ||
Recrystallisation inhibition (RI)
This is the property of AF(G)Ps to inhibit the rate of growth of an ice crystal, and not the ability to either suppress the freezing temperature or inhibit nucleation. Ice recrystallisation occurs between adjacent ice crystals, whereby the larger crystals grow at the expense of smaller ones in order to minimise the total surface energy, which can be considered to be a type of Ostwald ripening. In the presence of a recrystallisation inhibitor the ripening is either slowed or stopped. This is most conveniently measured by using the ‘splat’ test which allows for quantitative assessment of RI activity,15 but other methods have also been suggested.16 An example of splat tested RI inhibitor is shown in Fig. 1. Fig. 1E shows a PBS control allowed to anneal at −6 °C for 30 minutes, and Fig. 1F shows the same sample in the presence of an RI inhibitor which clearly has reduced grain sizes. In order to avoid false positive results in this assay it is essential to include salt (normally NaCl) in the solution.15 This allows the assay to be undertaken at a temperature above the eutectic point (−6 °C is the standard annealing temperature), ensuring the presence of liquid between ice crystal boundaries. This ensures that materials without RI behaviour cannot absorb at the ice/water interface, whereas in pure water significant absorption is possible, giving rise to false positive results. Quantitative assessment of ‘splat’ assayed AF(G)P requires the measurement of many individual ice grains within a polycrystalline wafer, which is both tedious and prone to error. Jackman et al. have developed software tools to facilitate this analysis and move it towards a high-throughput technique.17Other methods
Several other methods have been used to investigate the activity of AFGPs. However, the relative contribution of the above effects is often difficult to separate. For example, monitoring the freezing process by DSC gives a wealth of information and reveals additional transitions which can be heating/cooling rate dependent and also depression of freezing transitions (different to TH).18 These are not omitted because they are not informative, but rather to provide focus on three key features which the author believes are necessary to allow comparison of antifreeze properties between different materials as the outcomes of the tests can be clearly defined, which is essential for the main aim of this review, which is to establish the potential of synthetic polymers as AFGP mimics.Mechanism of action
Despite over 30 years of study of AF(G)Ps there is still no conclusive description of the mechanism by which they produce their different antifreeze properties, and also the large structural differences between the AFPs and AFGPs may suggest different interactions between the protein and ice surfaces. However, the generally accepted mechanism is that the AF(G)Ps can bind to a specific plane (or planes) of a growing ice crystal. Ice crystal growth then occurs between adjacent proteins resulting in a curved surface. As it is energetically unfavourable for further water molecules to add to a curved surface (due to the Kelvin effect) there is a depression in the freezing point, without affecting the energetics of removal of water molecules (melting) from the ice surface hence resulting in a thermal hysteresis gap. This mechanism is questionable as it requires irreversible binding to the ice surface, which means that significant AFGP absorption would be expected even at low concentrations, which is not the case. A schematic of this is shown in Fig. 2. This issue was addressed by Hall and Lips who suggested that the AF(G)P do not bind irreversibly, based on observations that the free energy of absorption is close to zero.19 The authors suggest that the peptides only temporarily block the addition of water molecules and hence the antifreeze properties do not arise due to the accumulation of peptide at a particular surface of the ice crystal.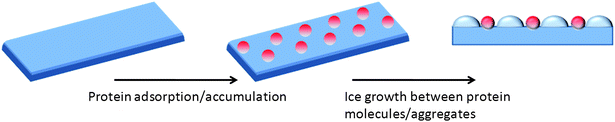 | ||
| Fig. 2 Simplified step pinning model for the mode of action of AFGPs on ice surface. Red spheres represent AFGP. Dark blue is ice wafer, and light blue the curved surface generated between adsorbed AFGP. | ||
An ice crystal held in a solution of AF(G)P, within the thermal hysteresis gap will tend to form hexagonal or hexagonal bipyramids, compared to circular crystals which are observed in the presence of other colligatively acting antifreezes (Fig. 1). This dynamic ice shaping effect indicates that the AF(G)Ps are acting on the surface of growing ice crystal lattices rather than within the bulk ice. Fig. 3A shows a schematic of hexagonal (1 h) ice. There are 2 basal faces which are perpendicular to the c-axis and 6 prism faces which are parallel to the c-axis. Under normal conditions ice growth occurs from the prism faces with only limited growth from the basal faces. The formation of hexagonal ice crystals in the presence of AF(G)P indicates that the prism faces are being inhibited. Hemispherical etching12 and observation of the formation of pits on ice crystals13 demonstrated that AFGP does indeed bind to the prism faces. NMR conformation studies indicate that the spacing between repeat units of the AFGP tripeptide (9.31 Å) matches two repeat units of ice along the prism faces (9.038 Å) suggesting that AFGP acts upon a complementary receptor site12 as is observed for many biological receptor–ligand interactions.20 Lowering the temperature further below the non-equilibrium freezing point results in significant growth from the basal planes giving rise to long thin “spicular” crystals with hexagonal bipyramidal symmetry (Fig. 1D). This is explained by an extension of the mattress model, called step pinning (Fig. 3B). When growth occurs from the basal face during overcooling a new prism face is also formed. AFGP can then absorb to this new prism face, preventing growth and hence giving rise to a basal face with less surface area than before. Continuing this many times results in the bipyramidal shape. Careful experiments with AFP where ice crystals were slowly cooled and warmed allowed for direct observation of the ridges being formed by sequential formation of additional basal faces (Fig. 1C).21 It is appropriate to again distinguish from the hyperactive AFPs which bind to both basal and pyramidal faces, resulting in their enhanced TH activity (up to 6 °C).7
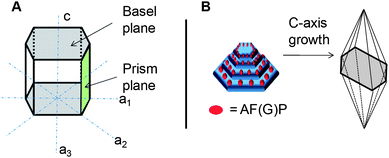 | ||
| Fig. 3 (A) Crystal structure of hexagonal ice including important crystallographic planes; (B) Formation of hexagonal bipyramidal crystals by inhibition of growth on prism faces due to absorption of AF(G)Ps. | ||
While this specific matching of hydrophilic residues to the ice crystal lattice is a convenient explanation, and has even been used for the rational design of new AFPs with predictable ice face recognition,22,23 there are still several questions outstanding. Most considerations of AF(G)P activity only consider the hydrophilic interactions between protein and ice, but there is increasing evidence that hydrophobic interactions are important,24,25 which would lead to significant entropic contributions by excluding addition of water from protein/ice surfaces.
It is often assumed that there is an abrupt interface between ice and water, which is not the case. Evidence suggests a region, around 10 Å thick where there is a gradual loss of ice structure before becoming bulk water.26 This is also known as the “quasi-liquid layer” (QLL) where water molecules are neither in a rigid solid structure nor in the liquid state. Recent evidence by Ben et al. using AFGP mimetic peptides (vide infra) suggests that recrystallisation-inhibition specific compounds (i.e. no TH activity) act at the bulk water–QLL interface,27,28 and that hydration of the ice-binding domains is a key parameter as the hydrating water molecules cannot become incorporated into the crystal lattice.29 The exact mechanisms remain under intense discussion, and the author points to several other articles which discuss this.8,30–33
Effect of AFGP structure on activity
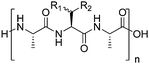
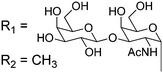
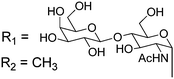
Owing to the relative structural simplicity of AFGP over AFP, AFGPs are an attractive target to create synthetic macromolecular mimics using modern polymer science. Hence from this point, the focus will be AFGPs, unless otherwise specified, to aid the discussion. Early structure–property relationship studies on AFGP demonstrated that oxidation of the hydroxyl groups of the carbohydrate to carboxylate groups removed all TH activity. Conversion of the C-6 hydroxyl to an aldehyde had a minimal effect on the TH. This suggests that a neutral moiety is required for tight ice-binding, but that minor variations in the carbohydrate might be tolerated.3 The most complete structure–property relationship study on AFGP was undertaken by Tachibana et al.34 It should be noted that in this work the authors only considered TH and dynamic ice shaping as an indication of antifreeze activity and no RI assays were undertaken. The first observation was that a single tripeptide motif could influence ice crystal growth, although there was no measurable TH, suggesting small peptides can be used for screening purposes. Furthermore, they observed that above 5 tripeptide repeat units there was no increase in the TH activity. Changing the stereochemistry of the anomeric linkage between peptide and carbohydrate from the native α to the synthetically more accessible β anomer resulted in no TH activity but interestingly, the ice shaping activity was maintained. Changing the internal glycosidic linkage in the disaccharide did not significantly affect the antifreeze properties (Table 1, A + B), and even exchange of the disaccharide for N-acetyl galactose retained most of the TH and ice shaping activity. The N-acetyl group at the C-2 position of the carbohydrate (both disaccharides and monosaccharides) was essential for TH activity, but ice shaping was maintained in its absence (Table 1, B + C). Circular dichroism spectroscopy revealed that the N-acetyl group also influenced the secondary structure, with peptides containing an acetamide group showing a polyproline type II structure and those without, a more disordered structure. Sialic acid derivative (with the N-acetyl group, Table 1, E) also had a polyproline(II) structure but had neither TH nor dynamic ice shaping activity. This suggests that a specific secondary structure and a specific ice recognition are essential for TH activity, but that the dynamic ice shaping is more tolerant of modifications, apart from the addition of charged groups. Serine substituted peptides (Table 1, D) also had no TH but had (different) helical structures. This evidence suggests that the TH activity can be effectively decoupled from ice shaping by maintaining the tight ice-binding of a carbohydrate unit, but with an altered secondary structure. This observation is significant as removing TH but retaining the RI is desirable for cryopreservation, and the reasons for this are discussed in the applications section below.| Code | Chemical structure | Conformationa | TH activeb | Ice shapingb |
|---|---|---|---|---|
| a Determined by CD spectroscopy. b Determined by use of a nanolitre osmometer. PPII denotes poly(L-proline) type II helix. Y = Property is observed; N = property is not observed. | ||||
|
||||
| A |
|
PPII | Y | Y (pyramidal) |
| B |
|
PPII | Y | Y (pyramidal) |
| C |
|
Disordered | N | Y (hexagonal) |
| D |

|
Disordered | N | N |
| E |
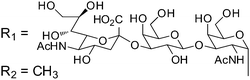
|
PPII | N | N |
| F |
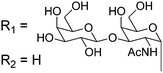
|
Helical | N | Y (hexagonal) |
The group of Ben has observed that a series of rather dramatic structural modifications (shown in Scheme 2) could lead to glycopeptides which displayed no measurable TH activity but maintained a remarkable level of RI activity35,36 which has also been seen in AFPs from Lolium perenne (ryegrass).37 Conformation analysis of peptide A in Scheme 2 by circular dichroism suggested a predominantly random coil structure, which is consistent with the results in Table 1, that TH inactive AFGP mimics have little or no long-range order.28 However, molecular dynamics simulations indicated that the carbohydrate folds back on the peptide (no intramolecular hydrogen bonding was observed by NMR) producing a hydrophobic pocket. Other peptides with either a longer or shorter spacer between the backbone and carbohydrate did not have this conformational feature, and also demonstrated little or no RI activity.
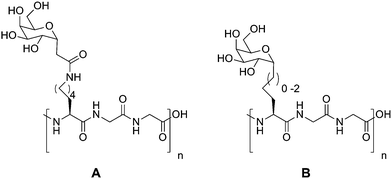 | ||
| Scheme 2 Synthetic AFGP mimics of Ben et al. | ||
In order to explain the specific recrystallisation inhibition activity of some AFGP mimics Ben et al. have proposed using the hydration index as a predictive tool.27 This was defined as being the number of tightly bound water molecules divided by the molar volume of the carbohydrate. Using a series of monosaccharides, and anomeric α-C-allylated monosaccharides, this index was found to correlate well with the observed recrystallisation inhibition activity as determined by the ‘splat’ assay. None of these monosaccharides could induce dynamic ice shaping, implying that they are not directly binding and inhibiting the ice crystal itself. Therefore, Tam et al. suggested that the carbohydrates and glycopeptides are instead acting at the quasi-liquid layer—the interface between bulk ice and water. This is reinforced by TEM evidence showing that trehalose (a disaccharide) can inhibit ice crystal growth better than other disaccharides (maltose and sucrose), which correlates well with their relative degrees of hydration, but rather high (15 wt%) concentrations of carbohydrate are used making direct comparisons to AFGP difficult.29 During freezing, trehalose is excluded from the ice crystal (as ice forms chemically homogeneous crystals) therefore increasing the concentration of trehalose, and hence increasing the concentration of hydrated (associated with carbohydrate) water molecules. This lowers the number of free H2O molecules thus preventing further ice growth. It is speculated that only free water molecules (i.e. those not associated with the carbohydrate) can add to ice crystals, therefore following freezing the concentration of water decreases. Trehalose is also commonly used as a cryoprotectant for protein pharmaceuticals, where it can both protect proteins during lyophilisation and increase their thermal stability.38 These effects appear to be related to their effect on the structure of water, but drawing direct comparisons is difficult. Fig. 4 shows a schematic illustrating a carbohydrate or glycopeptide at the QLL interface. Although this explanation fits for carbohydrates, the importance of the peptide unit must still be explained. It seems that a hydrophobic face must be present in some form for highly active RI behaviour. Most likely, the true mechanism is a balance of these, with the correct hydration being necessary to interface with the QLL and the presentation of these along a peptide backbone enhancing the effect. Both the mechanism of AFGP action and the nature of the QLL are topics undergoing much discussion.39 Finally, although there is currently no crystal structure of a native AFGP, there are several examples of them for AFP's. A key observation is that structurally different proteins, such as the alpha helical AFP ‘HPLC 6’5 or β-helical spruce budworm AFP40 both present a hydrophilic, threonine-rich, ice-binding face and an opposing hydrophobic face indicating that presentation of a hydrophobic face is a key feature. Combined 2-D NMR/molecular modelling studies by Nishimura et al.34 (and more recently by Ben et al.28) on TH active AFGP mimics indicated a similar amphipathic conformation suggesting that this feature is essential in both AFP and AFGP. These are shown in Fig. 5.
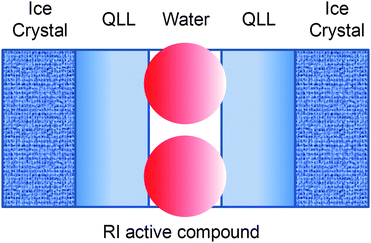 | ||
| Fig. 4 QLL = Quasi-liquid layer. | ||
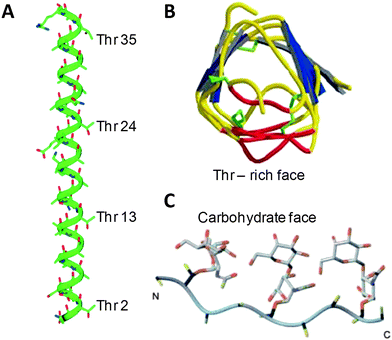 | ||
| Fig. 5 3-D structures of antifreeze-active peptides/proteins. (A) X-Ray crystal structure of HPLC 6; (B) X-Ray crystal structure of spruce budworm AFP; (C) Energy optimised solution structure of AFGP mimetic peptide A in Table 1. (A) and (C) reproduced with permission from Wiley-Blackwell, (reference 5 and 34 respectively) Copyright 1999 and 2004. (B) reproduced with permission from Macmillan Publishers Ltd: Nature (reference 40), Copyright 2000. | ||
Section summary
Considering this evidence a few general observations between the structure of AFGP mimics and their activity can be drawn.(1) Addition of charged functional groups into AFGP structures (not always true for AFP) is not tolerated, reducing both TH and RI activity.
(2) Tight ice-binding through the correct stereochemistry/orientation/spacing of the carbohydrate is important.
(3) High degrees of solvation of the carbohydrate residues are required for RI activity.
(4) Long range order in the inhibitor is important for TH activity but not so for RI activity.
(5) The presence of a hydrophobic face/pocket/region is essential for TH, RI and ice shaping activity.
From these 5 (generalised) observations it seems that synthetic polymers can provide most of the requirements for RI activity, but not for TH, particularly the long range order as although stereoregular polymers are known, they are less common and tend to not have the precise sequence control which allows presentation of moieties along a particular face of a helix or sheet-like structure. However, amphipathic synthetic polymers (i.e. containing a hydrophobic and hydrophilic face under certain conditions) have attracted much interest as mimics of the facially active host-defence peptides.41 The remainder of this review will focus on polymers which have been indicated to have some specific antifreeze behaviour.
Synthetic polymers with “antifreeze” activity
So far, only peptide-based materials have been discussed. While these have shown great promise the use of peptide/proteins for many applications can be limited by several factors. Obtaining large quantities of proteins is non-trivial, especially in the case of glycoproteins, as this prevents bacterial expression being used. Solid phase synthesis can be used, but again on a laboratory scale only limited quantities can be produced. Immunogenicity/toxicity must be considered and peptides/proteins require extensive testing for any detrimental in vivo effects. Synthetic polymers can overcome these problems and also have the benefit of being highly tuneable with respect to chemical composition, architecture and molecular weight. Recent advances in controlled/“living” radical polymerisations42–44 and highly efficient reactions for post-polymerisation modification45 (including the alkyne/azide cyclo-additions46) allow access to a huge range of synthetic polymers with predictable molecular weights, architecture and side chain functionality. Controlled radical polymerisation also allows access to hybrid materials containing short peptide sequences incorporated as blocks or side chains of synthetic polymers.47 There has been relatively little work into the area of functional polymers which can mimic the properties of AF(G)Ps, despite the huge academic and industrial interest in them, and also the high level of interest in using polymers or small molecules to control biomineralisation.48,49 This is most likely due to the expectation that a 3-dimensional structure with precise presentation of the ice-binding motifs is essential for antifreeze activity. As discussed in the previous section, this can in part be attributed to the assumption that TH is the only antifreeze property, and the prevailing literature suggests that precise structures are essential for, or at least enhance, this effect. However, work demonstrating the potential of RI active compounds in the field of polymer antifreezes has grown recently. This section will summarise the polymers which have shown antifreeze properties and attempt to compare the magnitude of these, although this is complicated by a lack of standard testing protocols being employed. All the polymers are summarised in Table 2, and their chemical structure are shown in Fig. 6.| Polymer | Codea | RI (splat)b | RI (optical)c | DSCd | TH/°Ce | Ice shaping (optical)f | Ice shaping (WAXS)g | Ref |
|---|---|---|---|---|---|---|---|---|
| a Structures in Fig. 5. b Recrystallisation inhibition quantified by “splat” assays. c Recrystallisation inhibition demonstrated. d Changes in crystallisation temperature measured by differential scanning calorimetry. e Thermal hysteresis gap magnitude. f Dynamic ice shaping as observed by single crystal growth methods. g Observations of change in crystal habit determined by wide angle X-ray scattering. | ||||||||
| PEG113-block-hB(PEI–Gly)23 | C | 3.1 °C depression, (1 mg mL−1) | 0.83 (1 mg mL−1)57 | Y57 Strong 002 disappears, 100, 101 broaden | 57 | |||
| hB(PEI–Gly)23 | B | 0.6 °C depression (1 mg mL−1) | 0.69 (1 mg mL−1)57 | Y57 Weak | 57 | |||
| PVA (various molecular weights) | A | Y53 | 0.024 (50 mg mL−1)54 | Y55 | 15,50,53,55,78,79 | |||
| PEO-block-HEE | E | Y | 1 °C depression, (2.5 mg mL−1) | Y | Y (002 disappears 100, 101 broaden and shift) | 14 | ||
| PEO-block-HEE–OPO3H | F | 3 °C increase, (2.7 mg mL−1) | Y (002 weakens 100, 101 shift) | 14 | ||||
| Poly(tartar amide) imine | H | Y | 0.2 °C depression (20 mg mL−1) | Y (002 disappears 101 weakens and splits) | 56 | |||
| Poly(tartar amide) PEG | G | 1.7 °C depression (20 mg mL−1) | Y (002 disappears 101 weakens and splits) | 56 | ||||
| PGalEMA | K | Y | 50 | |||||
| PGluEMA | J | Y | 50 | |||||
| PHyP | D | Y | 15,50 | |||||
| PAA·NH4 | L | 0.03 °C (300 mg mL−1) | Basal plane absorption | 58 | ||||
| PAA·NH4/AFP–I | L | 1 °C (300 mg mL−1) | Basal plane absorption | 80 | ||||
| PLL–COOH | Y | Y | 59 | |||||
| P(Man-Xyl) | I | 3.7 °C (5 mg mL−1) | 81 | |||||
| P(Lys-co-Succinate) | M | Y | Y | 59 |
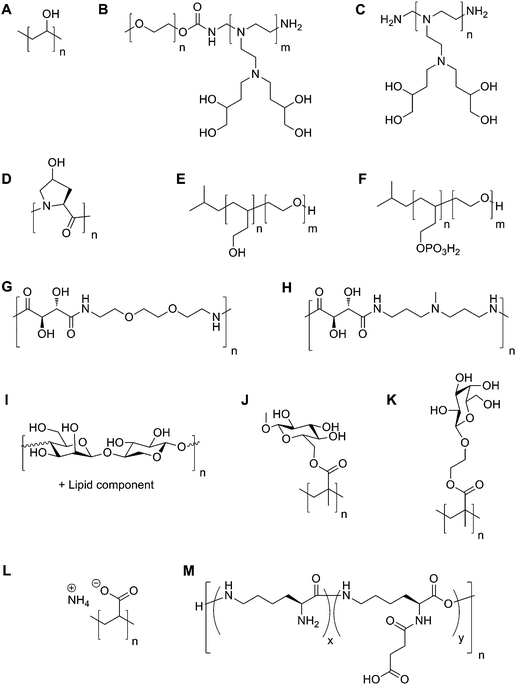 | ||
| Fig. 6 Chemical structures of polymers indicated to have specific antifreeze properties. | ||
The first observations that synthetic macromolecules could inhibit ice recrystallisation were made by Knight et al. in 1995.15 They evaluated 4 polypeptides and 3 vinyl-polymers for RI activity. In particular they showed that poly(L-histidine), poly(L-hydroxyproline) (E) and poly(vinyl alcohol) (A) all showed RI activity in the dilute (<1 mg mL−1) concentration range. These results are rather remarkable given the significant structural differences between each of these polymers, and to native AF(G)Ps. For example poly(L-hydroxyproline) (E) displays a helical structure, as do many AFPs,5 but it is not facially amphiphilic: rather the hydroxyl groups are distributed regularly along the whole backbone. Poly(L-histidine) an interesting case, as all (with the exception of M) the polymers in Table 2 (discussed more below) which show RI activity have neutral side chains, in particularly alcohol units. A recent study by Gibson et al.50 showed that poly(L-glutamic acid) and poly(L-lysine), prepared by NCA polymerisation,51 showed no RI behaviour. As these both have helical structures at physiological pH, as with poly(L-hydroxyproline) (E), it was concluded that charged side-chains do not interface with the growing ice-crystals correctly to induce TH. It should be noted that there are differences between the poly-proline type helix and the standard α-helix. In the case of poly(L-histidine) there will only be a partial positive charge at physiological pH, perhaps explaining its RI activity. The authors also compared the related vinyl-backbone polymers for RI activity and found the same trend that polymers with charged side chains do not give RI behaviour. It also seems that the side chain must be able to both donate and accept hydrogen bonds, explaining why poly(ethylene glycol) does not demonstrate RI. Gibson et al. finally evaluated the RI activity of synthetic glycopolymers52 with either β-D-glucose (J) or β-D-galactose (K) in the side chain. These polymers did display measurable RI behaviour, but it was rather small compared to poly(vinyl alcohol) (A). It has been shown that for AFGP-mimetic peptides, the distance between the carbohydrate and the peptide backbone is key, perhaps explaining the reduced activity.28,36 This study effectively demonstrated that having a polymer with hydroxyl side chains is not sufficient to guarantee strong RI activity, and that several other structural features must be taken into account. Inada and co-workers have extensively investigated the RI behaviour of PVA. Using the ‘splat’ assay, it was demonstrated that PVA (atactic) displays molecular weight dependent RI (higher Mw gives more RI) and that PVA with Mn of ∼90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 gives RI activity comparable to that of AFP type 8 on a molar level—it is important to note that the mass of PVA required is still significantly higher than for an AFP.53 Nonetheless, this is remarkable considering PVA has very few structural similarities to AFGPs. Single crystal growth experiments also revealed that PVA demonstrates small, but measurable, TH activity54 and dynamic ice shaping behaviour55 indicating that some specific interactions with a crystal face are occurring, with absorption occurring on the prism planes. Budke and Koop suggest that the spacing of hydroxyl groups of PVA are closely matched to that of the prism planes of hexagonal ice, with 3 PVA units (7.56 Å) having a similar spacing as every second O atom (7.36 Å) in the ice unit cell.55
000 g mol−1 gives RI activity comparable to that of AFP type 8 on a molar level—it is important to note that the mass of PVA required is still significantly higher than for an AFP.53 Nonetheless, this is remarkable considering PVA has very few structural similarities to AFGPs. Single crystal growth experiments also revealed that PVA demonstrates small, but measurable, TH activity54 and dynamic ice shaping behaviour55 indicating that some specific interactions with a crystal face are occurring, with absorption occurring on the prism planes. Budke and Koop suggest that the spacing of hydroxyl groups of PVA are closely matched to that of the prism planes of hexagonal ice, with 3 PVA units (7.56 Å) having a similar spacing as every second O atom (7.36 Å) in the ice unit cell.55
Börner and co-workers synthesised poly(tartar amides) (G + H) and investigated their ability to interact with ice.56 Some RI activity was implied through freezing assays but no quantitative evaluation was undertaken. The backbone spacer appeared to influence the properties of the polymers as judged by DSC and WAXS. The polymer with PEG spacers (G) gave a 1.7 K decrease in the crystallisation temperature of pure water, compared to only 0.2 K for a tertiary amine containing spacer (H). WAXS analysis showed that the (002) peak of hexagonal ice was significantly reduced upon addition of PEG, which is known to not display any TH or RI activity. PEG, however, did not block the (101) diffraction, but upon application of either of polymers G or H the peak was significantly reduced indicating some specific interactions with the ice due to the addition of the polyol segment. Poly(ethylene oxide)-block-poly[2-(2-hydroxyethyl)ethylene] (E) is another hydroxyl side chain linear polymer which had been suggested to have antifreeze properties.14 Although not evaluated quantitatively, this polymer was demonstrated to have strong recrystallisation inhibition and dynamic ice shaping effects. DSC experiments indicated a decrease in the onset of crystallisation, but TH was not measured using a nanolitre osmometer. As the constituent homopolymers were not investigated in this study it is not clear how each contributes to the observed effects, or if the PEG chain is necessary. Phosphorylation of the hydroxyl groups (F) removed the ice-crystal morphology changes, implying that hydroxyl groups were essential for complete inhibition of a crystal face, but RI/TH activity was not measured. Another hydroxylated block copolymer, poly(ethylene oxide)-block-poly(ethylene imine-glycidol adduct) (B) also displayed some antifreeze properties including crystal face modification.57 Impressively, the block copolymer displayed a thermal hysteresis gap of 0.83 °C which is comparable to native AFGPs. Without the PEG block (C) the TH gap decreased to 0.69 °C. Both these measurements were undertaken at constant mass concentration making direct comparison of efficacy difficult. Again, it is not clear if it is the nature of the PEG chain, or simply the increased molecular weight of the polymer (which is known to enhance both TH and RI in native AFGP) which enhances the effect. The disparity between DSC and osmometry data is shown here—by DSC the homopolymer showed a larger decrease in the crystallisation temperature relative to the block copolymer. Careful studies by Inada et al. have demonstrated that poly(acrylic acid) ammonium salt (L) has a weak, but measurable thermal hysteresis effect of only 0.04 °C, which was attributable to absorption onto the basal face of the growing ice crystal.58 In order to enhance the TH activity, the authors added AFP type 1 which specifically binds to the pyramidal planes. The resulting solute mixture had a far higher TH gap than the individual components, indicating a cooperative mechanism whereby each macromolecule blocks a different potential growing face of the ice crystal, which is analogous to mechanism of “hyperactive AFPs”. This method is promising as it can reduce the amount of the (expensive) AFP required to affect a strong TH response.
Despite this wide range of synthetic polymers with demonstrated antifreeze properties, there is only a single report on the cryopreservation of cells using specific polymeric RI inhibitors, to the best of our knowledge. Poly(ε-L-lysine) modified with between 0 and 100% of carboxylic acid groups (M) (through reaction with succinic anhydride) has been studied by Matsumura and Hyon.59 When 65% of the lysine repeat units were modified with carboxylic acids, the polymer was shown to modify the morphology of single ice crystals to give hexagonal shapes, as opposed to the spherical shapes seen for higher or lower degrees of carboxylic acid functionality. Furthermore, qualitative RI assays suggest that it also inhibited recrystallisation strongly at 65% functionality, indicating that there is a specific ratio of amines to acids required for activity. Due to the reduction in net positive charge along the backbone, the polymers' cytotoxicity was reduced at higher COOH content. Consequently, cryopreservation tests using L929 cells could be conducted at high polymer concentrations (up to 20 wt%). Following 6 hours of cryopreservation and thawing at 37 °C a significant increase in viable cells were observed using the copolymer with 50–70% COOH side chains relative to DMSO as model cryoprotectant. This is discussed further in the application section below. The underlying reasons for the strong activity of these polymers are still unclear. For comparison, the authors used poly(acrylic acid) modified with 5% NH2 groups. This polymer also showed some (though not as potent as the poly(lysine)-based materials) cryoprotective properties, while the native PAA homopolymer had very little. These results suggest that poly(ampholytes) are strong candidates for future cryopreservation agents and also suggest that it is possible to reproduce AF(G)P activity without hydroxyl groups as the ice-binding domains.
Potential applications
Synthetic materials which display specific antifreeze properties and control the structure of water during freezing have huge commercial and academic potential in a wide variety of fields.60 It is important to note that this is not limited to cryopreservation which is the most obvious application. Applications of AF(G)Ps which are not compatible with synthetic materials should also be mentioned, but will not be expanded on, include expression of the proteins in situ, for example to create freeze-tolerant crops or fish production in colder waters.61Cryopreservation implies enhancing long term viability of a biological sample through cooling the cells/tissue, thus slowing their intracellular degradation processes such that the timescale over which they are viable is increased—assuming successful thawing. This is a key challenge due to the current limits for the storage of donor organs for transplantation, and the ability to bank organs at source would greatly improve the situation. One possible solution is to use vitrification, where intracellular water is replaced with a glass forming solvent such as DMSO.62 While successful, these additives are often cytotoxic and require very controlled freezing/cooling cycles and may not be applicable to large organ samples.63 It has been noted that the major cause of cellular damage in cryopreservation is the recrystallisation of ice during thawing.64,65 Reducing the rate of ice crystal growth during this period would be expected to significantly improve the viability of the cryopreserved samples and was recently demonstrated by Matsumura et al. for rat mesenchymal stem cells and murine L929 cells. Fig. 7 shows some results of the cryopreservation compared to a common cryoprotectant DMSO. These data indicate the polymers are more effective than DMSO (Fig. 7A), and that the cells are healthy post-thawing (Fig. 7B).59 A significant limitation of native AFGP is dynamic ice shaping/TH (assuming they are intrinsically related): during freezing/thawing native AFGP change the crystal habit to form long spicular ice crystals which cause mechanical damage to cells resulting in increased cellular damage. Red blood cells and islet cells have been successfully cryopreserved with AFGPs and a synthetic mimic, but the maximum concentration which can be applied (and hence maximum cryoprotective effect) is limited by the onset of increased cell damage, presumably due to the spicular ice.66,67 Mouse sperm cell viability was decreased post-cryopreservation at all concentrations of AFGP indicating that subtle differences between cells can modulate the potential of AFGPs.68 In 2008 Amir et al. used AFP solutions to lower the freezing temperature of preservation solutions for rat hearts.69 This method allowed the hearts to be stored at temperatures lower than normal (4 °C is normally used for organ storage) without the solution freezing. Following transplantation, the hearts stored in the presence of AFP survived transplantation, while half died without the AFP.
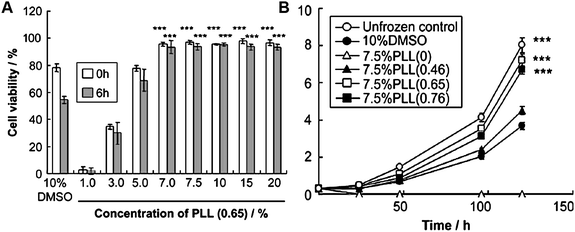 | ||
| Fig. 7 Cryopreservation of L929 cells using polymer M, with 65% succinic acid functionality. (A) Cell viability following cryopreservation with various concentrations of M immediately (white bars) and 6 hours after (grey bars) thawing; (B) cell proliferation assay following cryopreservation with M compared to DMSO. Charts are reprinted fromreference 59, Copyright (2009), with permission from Elsevier. | ||
The final cryoprotective benefit of AF(G)Ps is the stabilisation of membranes during cooling—phospholipid membranes display a phase transition between 4 and 12 °C typically. Below this temperature the membranes can leak their contents, which in a biological setting leads to cell death. Experiments with model vesicles demonstrated that addition of AFGP reduced the amount of dye leakage during cooling from room temperature to 0 °C (i.e. above freezing temperatures) indicating a direct interaction between the proteins and the membrane.70,71 This is shown in Fig. 8, which also indicates that higher molecular weight AFGPs confer increase protection to the cell membranes.
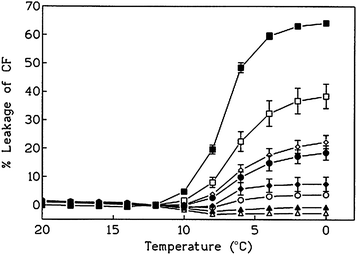 | ||
| Fig. 8 Leakage of carboxyfluorescein from dielaidoylphosphatidylcholine liposomes during cooling from 20 to 0 °C at 25 µg mL−1.■ = control (no AFGP), ● = ∼3.4 kDa, □ = 2.6, ◇ = 3.9 kDa, ▲ = 6–10 kDa, ○ = ∼13 kDa, ◆ = 21.7 kDa, △ = 24 kDa. Chart is reprinted from reference 71, Copyright (2001), with permission from Elsevier.71 | ||
Cryosurgery is a technique which relies on cryogenically cooling localised regions of unhealthy tissue (e.g. cancer) to induce cell death.72 Rubinsky and co-workers have taken advantage of the negative effect of AFPs during cryostorage (i.e. addition of AFPs reduces cell viability due to uncontrolled ice growth from basal faces) to enhance cryosurgery.73 Addition of AFPs to liver tissue during freezing resulted in significantly higher levels of cell death than in its absence, with a particularly large increase (>2 fold) when two freeze–thaw cycles were used.
AF(G)P mimics have been implicated as potential additives in frozen food.74 Soft fruits such as strawberries freeze very poorly due to cellular destruction, and ‘freezer burn’ affects meat when stored for extended periods of time. Injection of AFGP into lambs before slaughter gave rise to reduced ice crystal sizes in the meat following freezing and improved texture.75 It is noted in this report that this method may not be a consumer tolerated method to improve freeze-storage of meat. Frozen food (such as ice cream) also benefits from the addition of compounds which reduce ice recrystallisation as this improves the texture. Certain AFPs, expressed in yeast, are already being used in low fat frozen products. Importantly, the proteins must also be heat stable, which allows for high temperature processing during food preparation, which may limit other protein-based strategies. These proteins appear to be non-allergenic76 and non-toxic,77 but this must be a key consideration for any new materials. Inada and Modak have suggested that AF(G)P mimics can be applied in ice slurries, used to transport “cold energy”, by reducing agglomeration and thus reducing blockages.78
An important consideration for all the above applications is not only the absolute magnitude of an individual antifreeze property, but the amount of material required to induce this effect which is a key consideration for translating into an industrial application. For example, polymer M (Table 2 and Fig. 5) requires a 7 weight percent solution, equal to 70 grams per litre, for effective cryopreservation, or polymers K and J, which require 4 weight percent to have clearly discernible RI behaviour. These are in contrast to the mimics of Nishimura et al., which had maximal cryopreservation effects at only 0.5 weight percent. The contrasting results again highlight the need for quantitative analysis of the physico-chemical parameters (RI, TH, DIS) of any new materials (vide infra), including consideration of their dose-dependent behaviour, which may smooth the transition from academic to industrial application.
Future prospects and challenges
This review article is intended to introduce the field of AF(G)Ps to the polymer science community with particular regard to how modern polymer science might address the inherent difficulties associated with using the native materials. The results presented above demonstrate the strong academic and industrial interest in the development of AF(G)P-based materials. The author believes there are several key questions which still need to be addressed within the field:(1) Additional information on the molecular-level mechanisms of action and suitable explanations for the separation of RI and TH activities is required.
(2) What is the influence of long-range order along the polymer/peptide backbone on activity?
(3) What functional groups can be used for the ice-binding motifs, and what additional functional groups can be tolerated?
(4) Can polymeric materials be designed/synthesised with specific RI but not TH activity?
(5) Do specific polymer-based recrystallisation inhibitors improve cryopreservation of a wide-range of cells lines?
(6) How can point 5 be extended to tissue and organ samples?
Finally, the author believes that synthetic polymers can address these issues and it is a potentially fertile area of research for biotechnological and biomedical fields, as indicated by the range of structures in Table 2 and Fig. 6. However, this is dependent upon a clear appreciation of the different macroscopic properties: RI, TH and ice-shaping. The author recommends that all three of these properties are evaluated with new materials to ensure comparisons can be made not only between polymers, but the native proteins also. This is essential if structure–activity relationships are to be drawn.
Acknowledgements
MIG acknowledges the Birmingham Science City: Innovative Uses for Advanced Materials in the Modern World (West Midlands Centre for Advanced Materials Project 2), with support from Advantage West Midlands (AWM) and part funded by the European Regional Development Fund (ERDF) for an Independent Research Fellowship. MIG is also grateful to the University of Warwick and the Royal Society for funding his ongoing research.References
- A. DeVries and D. E. Wohlschlag, Science, 1969, 163, 1073–1075 CAS.
- A. L. DeVries, S. K. Komatsu and R. E. Feeney, J. Biol. Chem., 1970, 245, 2901–2908 CAS.
- M. M. Harding, P. I. Anderberg and A. D. J. Haymet, Eur. J. Biochem., 2003, 270, 1381–1392 CrossRef CAS.
- K. V. Ewart, Q. Lin and C. L. Hew, Cell. Mol. Life Sci., 1999, 55, 271–283 CrossRef CAS.
- M. M. Harding, L. G. Ward and A. D. J. Haymet, Eur. J. Biochem., 1999, 264, 653–665 CrossRef CAS.
- D. Worrall, L. Elias, D. Ashford, M. Smallwood, C. Sidebottom, P. Lillford, J. Telford, C. Holt and D. Bowles, Science, 1998, 282, 115–117 CrossRef CAS.
- A. J. Scotter, C. B. Marshall, L. A. Graham, J. A. Gilbert, C. P. Garnham and P. L. Davies, Cryobiology, 2006, 53, 229–239 CrossRef CAS.
- Y. Yeh and R. E. Feeney, Chem. Rev., 1996, 96, 601–618 CrossRef CAS.
- R. E. Lee and J. P. Costanzo, Annu. Rev. Physiol., 1998, 60, 55–72 CrossRef CAS.
- Gurian-Sherman and S. E. Lindow, FASEB J., 1993, 14, 1338–1344.
- A. Chakrabartty, D. S. C. Yang and C. L. Hew, J. Biol. Chem., 1989, 264, 11313–11316 CAS.
- C. A. Knight, E. Driggers and A. L. DeVries, Biophys. J., 1993, 64, 252–259 CAS.
- J. A. Raymond, P. Wilson and A. L. DeVries, Proc. Natl. Acad. Sci. U. S. A., 1989, 86, 881–885 CAS.
- Y. Mastai, J. Rudloff, H. Cölfen and M. Antonietti, ChemPhysChem, 2001, 1, 119–123.
- C. A. Knight, D. Wen and R. A. Laursen, Cryobiology, 1995, 32, 23–34 CrossRef CAS.
- M. M. Tomczak, C. B. Marshall, J. A. Gilbert and P. L. Davies, Biochem. Biophys. Res. Commun., 2003, 311, 1041–1046 CrossRef CAS.
- J. Jackman, M. Noestheden, D. Moffat, J. P. Pezacki, S. Findlay and R. N. Ben, Biochem. Biophys. Res. Commun., 2007, 354, 340–344 CrossRef CAS.
- T. N. Hansen and J. F. Carpenter, Biophys. J., 1993, 64, 1843–1850 CrossRef CAS.
- D. G. Hall and A. Lips, Langmuir, 1999, 15, 1905–1912 CrossRef CAS.
- L. E. Limbird, Mol. Interventions, 2004, 4, 326–336 CrossRef CAS.
- M. Griffith and M. W. F. Yaish, Trends Plant Sci., 2004, 9, 399–405 CrossRef CAS.
- M. J. Kuiper, J. V. Fecondo and M. G. Wong, J. Pept. Res., 2002, 59, 1–8 CrossRef CAS.
- W. Zhang and R. A. Laursen, FEBS Lett., 1999, 455, 372–376 CrossRef CAS.
- H. Chao, M. E. J. Houston, R. S. Hodges, C. M. Kay, B. D. Sykes, M. C. Loewen, P. L. Davies and F. D. Sönnichsen, Biochemistry, 1997, 36, 14652–14660 CrossRef CAS.
- J. Baardsnes and P. L. Davies, Biochim. Biophys. Acta, Proteins Proteomics, 2002, 1601, 49–54 CrossRef CAS.
- O. A. Karim and A. D. J. Haymet, J. Chem. Phys., 1988, 89, 6889–6896 CrossRef CAS.
- R. Y. Tam, S. S. Ferreira, P. Czechura, J. L. Chaytor and R. N. Ben, J. Am. Chem. Soc., 2008, 130, 17494–17501 CrossRef CAS.
- R. Y. Tam, C. N. Rowley, I. Petrov, T. Zhang, N. A. Afagh, T. K. Woo and R. N. Ben, J. Am. Chem. Soc., 2009, 131, 15745–15753 CrossRef CAS.
- T. Uchida, M. Nagayama, T. Shibayama and K. Gohara, J. Cryst. Growth, 2007, 299, 125–135 CrossRef CAS.
- C. L. Hew and D. S. C. Yang, Eur. J. Biochem., 1992, 203, 33–42 CAS.
- J. D. Madura, K. Baran and A. Wierzbicki, J. Mol. Recognit., 2000, 13, 101–113 CrossRef CAS.
- E. Kristiansen and K. E. Zachariassen, Cryobiology, 2005, 51, 262–280 CrossRef CAS.
- S. Zepeda, E. Yokoyama, Y. Uda, C. Katagiri and Y. Furukawa, Cryst. Growth Des., 2008, 8, 3666–3672 CrossRef CAS.
- Y. Tachibana, G. L. Fletcher, N. Fujitani, S. Tsuda, K. Monde and S.-I. Nishimura, Angew. Chem., Int. Ed., 2004, 43, 856–862 CrossRef CAS.
- A. Eniade, M. Purushotham, R. N. Ben, J. B. Wang and K. Horwarth, Cell Biochem. Biophys., 2003, 38, 115–124 CrossRef CAS.
- S. Liu and R. N. Ben, Org. Lett., 2005, 7, 2385–2388 CrossRef CAS.
- C. Sidebottom, S. Buckley, P. Pudney, S. Twigg, C. Jarman, C. Holt, J. Telford, A. McArthur, D. Worral, R. Hubbard and P. Lillford, Nature, 2000, 406, 256 CrossRef CAS.
- J. K. Kaushik and R. Bhat, J. Biol. Chem., 2003, 278, 26458–26465 CrossRef CAS.
- K. A. Sharp and J. M. Vanderkooi, Acc. Chem. Res., 2010, 43, 231–239 CrossRef CAS.
- S. P. Greather, M. J. Kulper, S. M. Gagnes, V. K. Walker, Z. Jia, B. D. Sykes and P. L. Davies, Nature, 2000, 406, 325–328 CrossRef CAS.
- K. Lienkamp and G. N. Tew, Chem.–Eur. J., 2009, 15, 11784–11800 CrossRef CAS.
- S. Perrier and P. Takolpuckdee, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 5347–5393 CrossRef CAS.
- K. Matyjaszewski and J. Xia, Chem. Rev., 2001, 101, 2921–2990 CrossRef CAS.
- C. J. Hawker, A. W. Bosman and E. Harth, Chem. Rev., 2001, 101, 3661–3688 CrossRef CAS.
- M. A. Gauthier, M. I. Gibson and H.-A. Klok, Angew. Chem., Int. Ed., 2009, 48, 48–58 CrossRef CAS.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS.
- K. Heredia and H. D. Maynard, Org. Biomol. Chem., 2007, 5, 45–53 RSC.
- S. I. Stupp and P. V. Braun, Science, 1997, 277, 1242–1248 CrossRef CAS.
- S. Tugulu, M. Harms, M. Fricke, D. Volkmer and H.-A. Klok, Angew. Chem., Int. Ed., 2006, 45, 7458–7461 CrossRef CAS.
- M. I. Gibson, C. A. Barker, S. G. Spain, L. Albertin and N. R. Cameron, Biomacromolecules, 2009, 10, 328–333 CrossRef CAS.
- M. I. Gibson and N. R. Cameron, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 2882–2891 CrossRef CAS.
- S. G. Spain, M. I. Gibson and N. R. Cameron, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 2059–2072 CrossRef CAS.
- T. Inada and S.-S. Lu, Cryst. Growth Des., 2003, 3, 747–752 CrossRef CAS.
- T. Inada and S.-S. Lu, Chem. Phys. Lett., 2004, 394, 361–365 CrossRef CAS.
- C. Budke and T. Koop, ChemPhysChem, 2006, 7, 2601–2606 CrossRef CAS.
- Y. E. Yagci, M. Antonietti and H. G. Börner, Macromol. Rapid Commun., 2006, 27, 1660–1664 CrossRef CAS.
- E. Baruch and Y. Mastai, Macromol. Rapid Commun., 2007, 28, 2256–2261 CrossRef CAS.
- K. Funakoshi, T. Inada, T. Tomita, H. Kawahara and T. Miyata, J. Cryst. Growth, 2008, 310, 3342–3347 CrossRef CAS.
- K. Matsumura and S.-H. Hyon, Biomaterials, 2009, 30, 4842–4849 CrossRef CAS.
- S. Venketesh and C. Dayananda, Crit. Rev. Biotechnol., 2008, 28, 57–82 CrossRef CAS.
- H. M. Zbikowska, Transgenic Res., 2003, 12, 379–389 CrossRef CAS.
- G. M. Fahy, B. Wowk, J. Wu, J. Phan, C. Rasch, A. Chang and E. Zendejas, Cryobiology, 2004, 48, 157–178 CrossRef CAS.
- G. M. Fahy, J. Saur and R. J. Williams, Cryobiology, 1990, 27, 492–510 CrossRef CAS.
- T. Wang, Q. Zhu, X. Yang, J. R. Layne and A. L. DeVries, Cryobiology, 1994, 31, 185–192 CrossRef CAS.
- V. Bouvet and R. N. Ben, Cell Biochem. Biophys., 2003, 39, 133–144 CrossRef CAS.
- J. F. Carpenter and T. N. Hansen, Proc. Natl. Acad. Sci. U. S. A., 1992, 89, 8953–8957 CrossRef CAS.
- S. Matsumoto, M. Matsusita, T. Morita, H. Kamachi, S. Tsukiyama, Y. Furukawa, S. Koshida, Y. Tachibana, S.-I. Nishimura and S. Todo, Cryobiology, 2006, 52, 90–98 CrossRef CAS.
- C. Koshimoto and P. Mazur, Cryobiology, 2002, 45, 49–59 CrossRef CAS.
- G. Amir, B. Rubinsky, Y. Kassif, L. Horowitz, A. K. Smolinsky and J. Lavee, Eur. J. Cardio-Thorac. Surg., 2003, 24, 292–297 CrossRef.
- Y. Wu and G. L. Fletcher, Biochim. Biophys. Acta, Gen. Subj., 2000, 1524, 11–16 Search PubMed.
- Y. Wu, J. Banoub, S. V. Goddard, M. H. Kao and G. L. Fletcher, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2001, 128, 265–272 CrossRef CAS.
- R. Goel, K. Anderson, J. Slaton, F. Schmidlin, G. Vercellotti, J. Belcher and J. C. Bischof, J. Biomech. Eng., 2009, 131, 074003 CrossRef.
- H. Koushafar, L. Pham, C. Lee and B. Rubinsky, J. Surg. Oncol., 1997, 66, 114–121 CrossRef CAS.
- M. Griffith and K. V. Ewart, Biotechnol. Adv., 1995, 13, 375–402 CrossRef CAS.
- S. R. Payne and O. A. Young, Meat Sci., 1995, 41, 147–155 CrossRef CAS.
- B. Baderschneider, R. W. R. Crevel, L. K. Earl, A. Lalljie, D. J. Sanders and I. J. Sanders, Food Chem. Toxicol., 2002, 40, 965–978 CrossRef CAS.
- T. Hall-Manning, M. Spurgeon, A. M. Wolfreys and A. P. Baldrick, Food Chem. Toxicol., 2004, 42, 321–333 CrossRef CAS.
- T. Inada and P. R. Modak, Chem. Eng. Sci., 2006, 61, 3149–3158 CrossRef CAS.
- S.-S. Lu, T. Inada, A. Yabe, X. Zhang and S. Grandum, Int. J. Refrig., 2002, 25, 562–568 CrossRef CAS.
- K. Funakoshi, T. Inada, H. Kawabata and T. Tomita, Biomacromolecules, 2008, 9, 3150–3156 CrossRef CAS.
- K. R. Walters, A. S. Serianni, T. Sformo, B. M. Barnes and J. G. Duman, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 20210–20215 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
