Synthesis of nanotubes via cationic polymerization of styrene and divinylbenzene
Tanay
Kesharwani
,
Justin S.
Valenstein
,
Brian G.
Trewyn
,
Fengkui
Li
,
Victor S.-Y.
Lin
and
Richard C.
Larock
*
Department of Chemistry, Iowa State University, Ames, IA 50011, USA. E-mail: larock@iastate.edu; Fax: +1-515-2940105; Tel: +1-515-2944660
First published on 6th September 2010
Abstract
A simple method for the preparation of nanoscale fibers and tubes by the cationic copolymerization of divinylbenzene (DVB) and styrene (ST) is described.
Tubular structures1 are the most versatile module for electronic devices due to their ability to transport neutral, ionic and chiral species.2 Transport processes and chemical transformations can be coupled to afford a flow-through nanoreactor for advanced analytical applications.3
The synthesis of organic nanotubes has recently received much attention due to their potential applications, including extensive use in biological systems.4 There are four general strategies for the formation of organic nanotubes, namely formation of a helical backbone,5 the stacking of rings6 and rosettes,7 and cylindrical micelles or rolled sheets arising from curved bilayers of certain amphiphiles.8 All of these approaches use a combination of covalent and non-covalent interactions for the formation of tubular structures. Usually the increased stability is provided by defined chemical bonds, whereas the supramolecular interactions allow their facile synthesis.
Tubular structures can also be synthesized by using di- and triblock copolymers as amphiphiles.9 The synthesis of organic nanotubes by direct polymerization processes or self-assembly of ill-defined macromolecules has not received much attention. The ease of synthesis and commercial availability of the desired monomers makes this approach very attractive. However, it suffers the disadvantage of the limited predictability of the self-assembly process. Recently Yan and co-workers have reported stable and soluble tubes by the self-assembly of a hyper-branched, multi-arm copolymer.10
Several methods for the preparation of nanoscale spherical particles via precipitation polymerization have been reported.11 However, this method has not been utilized for the synthesis of nanotubes until recently.12 Herein, we report a more direct and simple method for the preparation of nanoscale fibers and tubes at room temperature by the cationic polymerization of divinylbenzene (DVB) and its copolymerization with styrene (ST).† In a typical experiment, a dilute solution of DVB in hexane (16.6 g L−1) is added to a round bottom flask equipped with a magnetic stirrer. Distilled grade boron trifluoride diethyl etherate initiator is added to the solution dropwise using a syringe. Typically, 5 parts of initiator per 100 parts of reactants have been used. The solution is allowed to stir at 500 rpm and room temperature. Solid particle-like polymers suspended in the stirring solution appear during addition of the initiator. The resulting polymer particles are observed to grow in number with time. The reaction is terminated after 6 h by stopping the stirring, and the resulting materials are allowed to settle to the bottom of the flask and are collected by filtration. The original yellow polymeric materials turn white upon washing with acetone. After vacuum drying, the isolated polymer yield is approximately 80%.
A SEM micrograph of the product indicates that the materials are actually a large number of clusters of fine fibers with closed ends (Fig. 1a and b). Upon TEM analysis, we have found that most of the single strands are not hollow (Fig. 1c and d). The diameter of these nanorods ranges from 300 to 400 nm.
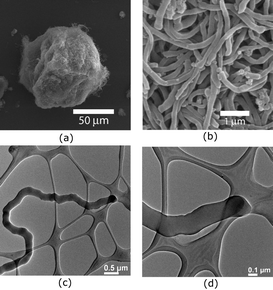 | ||
| Fig. 1 SEM (a and b) and TEM (c and d) images of fibers obtained from the polymerization of DVB in hexanes at 25 °C. | ||
We also examined the cationic polymerization of styrene as a co-monomer along with the commercially available DVB. We find that when 70 wt% of DVB is employed with 30 wt% of styrene, the SEM of the polymer remains unchanged (Fig. 2a and b) and does not show any opening at the end of the fiber. The thickness of the fibers is also unchanged and found to be in the same range as obtained previously with DVB, i.e. 300–400 nm. Also, TEM analysis indicates that the majority of the structures are solid rods with no openings. However, some of the fibrous strands have openings with diameters ranging from 5–30 nm (Fig. 2c and d).
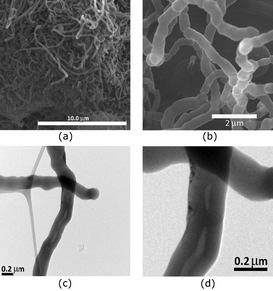 | ||
| Fig. 2 SEM (a and b) and TEM (c and d) images of fibers obtained from the polymerization of 70 wt% of DVB and 30 wt% of ST in hexanes at 25 °C. | ||
After the encouraging observation obtained when using 30 wt% of ST, we decided to increase the amount of ST to see what further changes in the morphology of the polymer might occur. To our surprise, increasing the amount of ST to 45 wt% results in a polymer with good morphological control as observed by SEM analysis (Fig. 3a–d). TEM analysis of these polymeric solid particles revealed that most of the tubes were hollow (Fig. 4a–d) with some blockage at the end and middle of the tubes. The diameter of the tubes ranges from 5–50 nm and the thickness of the tubes is found to be in the range of 200–300 nm.
 | ||
| Fig. 3 SEM images of fibers obtained from the polymerization of 55 wt% DVB and 45 wt% of ST in hexanes at 25 °C. | ||
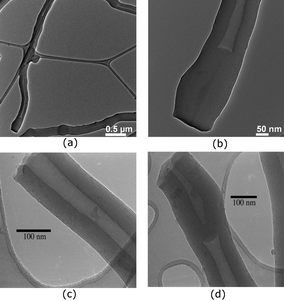 | ||
| Fig. 4 TEM images of fibers obtained from the polymerization of 55 wt% DVB and 45 wt% of ST in hexanes at 25 °C. | ||
Using up to 50 wt% of ST gave similar results (Fig. 5). However, no indication of open ended tubes was observed by SEM and most of the ends of the strands were blocked or showed some indentations (Fig. 5a and b). The TEM analysis of these elongated polymers, however, showed a hollow channel ranging in diameter from 5–50 nm (Fig. 5c and d). Some blockage at the ends of the tubes and in the middle of the tubes has also been seen. This might be the reason for not observing a hollow channel in the SEM analysis. One of the possible reasons for formation of the tubular structure may be the formation of a helical structure. However, the unpredictability of the shape and thickness can be attributed to the random nature of this polymerization process.
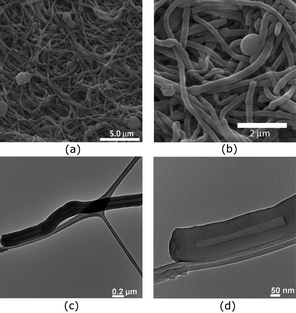 | ||
| Fig. 5 SEM (a and b) and TEM (c and d) images of fibers obtained from the polymerization of 50 wt% of DVB and 50 wt% of ST in hexanes at 25 °C. | ||
We also performed similar reactions with few substituted styrenes. Since we conducted the earlier experiments using a certain wt% of the substrates, we employed the same number of moles of the substituted styrene as styrene (0.0196 moles) and kept all the other parameters constant. p-Methylstyrene led to the formation of spherical particles (Fig. 6a and b). p-Methoxystyrene gave mostly an elongated material with a diameter of about 200 nm (Fig. 6c and d). However, we did not find any hollow openings in this material. These polymerization reactions were highly dependent upon temperature, solvent, stirring speed and concentration. We hope that in the future we will be able to optimize above mentioned parameters for these substituted styrenes which will lead to interesting morphologies.
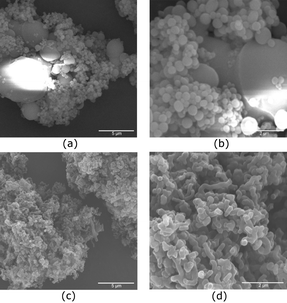 | ||
| Fig. 6 SEM images of polymer obtained from the polymerization of DVB with p-methylstyrene (a and b) and p-methoxystyrene (c and d). | ||
Conclusions
In summary, the simple approach presented here offers a promising method for the preparation of nanometre scale polymeric materials. The cationic polymerization of DVB in hexanes produces nanofibers with a diameter of 300–500 nm. The copolymerization of DVB with styrenes produces nanotubes with internal diameters ranging from 5–50 nm. Our initial effort to functionalize the tubes by incorporating substituted styrenes failed. However, encouraging results obtained in the case of p-methoxystyrene has led us to believe that substituted styrenes can provide exciting opportunities for the facile preparation of novel and useful nanomaterials. In future efforts will also be directed towards further fine tuning the morphologies of the resulting polymers by varying the stoichiometry, solvent, stirring mode and speed, and reaction temperature.Notes and references
- P. F. H. Schwab, M. D. Levin and J. Michl, Chem. Rev., 1999, 99, 1863–1933 CrossRef CAS.
- H. Lim, H. S. Shin, H. Shin and H. Choi, J. Am. Chem. Soc., 2008, 130, 2160–2161 CrossRef CAS; G. Chai, H. Heinrich, L. Chow and T. Schenkel, Appl. Phys. Lett., 2007, 91, 103101-1–103101-3; D. V. Bavykin and F. C. Walsh, J. Phys. Chem. C, 2007, 111, 14644–14651 CrossRef CAS; C. Gomez-Navarro, P. J. Pablo and J. Gomez Herrero, J. Mater. Sci.: Mater. Electron., 2006, 17, 475–482 CrossRef CAS; K. Malek and R. A. van Santen, J. Membr. Sci., 2008, 311, 192–199 CrossRef CAS; B. Eisenberg, Acc. Chem. Res., 1998, 31, 117–123 CrossRef CAS.
- C. Han, D. Hong, I. Kim, J. Gwak, S. Han and K. C. Singh, Sens. Actuators, B, 2007, 128, 320–325 CrossRef; M. Remskar, A. Mrzel, M. Virsek and A. Jesih, Adv. Mater., 2007, 19, 4276–4278 CrossRef CAS; F. Cao, K. Zhong, A. Gao, C. Chen, Q. Li and Q. Chen, J. Phys. Chem. B, 2007, 111, 1724–1728 CrossRef CAS; J. Hu, Y. Bando, J. Zhan, C. Zhi and D. Golberg, Nano Lett., 2006, 6, 1136–1140 CrossRef CAS.
- D. T. Bong, T. D. Clark, J. R. Granja and M. R. Ghadiri, Angew. Chem., Int. Ed., 2001, 40, 988–1011 CrossRef CAS.
- T. Nakano and Y. Okamoto, Chem. Rev., 2001, 101, 4013–4038 CrossRef CAS; D. J. Hill, M. J. Mio, R. B. Prince, T. S. Hughes and J. S. Moore, Chem. Rev., 2001, 101, 3893–4011 CrossRef CAS; J. J. L. M. Cornelissen, A. E. Rowan, R. J. M. Nolte and N. A. J. M. Sommerdijk, Chem. Rev., 2001, 101, 4039–4070 CrossRef CAS; R. R. Ketchem, W. Hu and T. A. Cross, Science, 1993, 261, 1457–1460 CAS.
- J. R. Granja and M. R. Ghadiri, J. Am. Chem. Soc., 1994, 116, 10785–10786 CrossRef CAS; N. Khazanovich, J. R. Granja, D. E. McRee, R. A. Milligan and M. R. Ghadiri, J. Am. Chem. Soc., 1994, 116, 6011–6012 CrossRef CAS; G. Gattuso, S. Menzer, S. A. Nepogodiev, J. F. Stoddart and D. J. Williams, Angew. Chem., Int. Ed. Engl., 1997, 36, 1451–1454 CAS.
- H. Fenniri, B. L. Deng and A. E. Ribbe, J. Am. Chem. Soc., 2002, 124, 11064–11072 CrossRef CAS; S. L. Forman, J. C. Fettinger, S. Pieraccini, G. Gottarelli and J. T. Davis, J. Am. Chem. Soc., 2000, 122, 4060–4067 CrossRef CAS; I. S. Choi, X. Li, E. E. Simanek, R. Akaba and G. M. Whitesides, Chem. Mater., 1999, 11, 684–690 CrossRef CAS.
- A. Mueller and D. F. O'Brien, Chem. Rev., 2002, 102, 727–757 CrossRef CAS; E. M. Wilson-Kubalek, R. E. Brown, H. Celia and R. A. Milligan, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 8040–8045 CrossRef CAS; J. M. Schnur, Science, 1993, 262, 1669–1676 CrossRef CAS; T. Shimizu, M. Masuda and H. Minamikawa, Chem. Rev., 2005, 105, 1401–1443 CrossRef CAS.
- G. J. Liu, Adv. Polym. Sci., 2008, 220, 29–64 CAS.
- D. Yan, Y. Zhou and J. Hou, Science, 2004, 303, 65–67 CrossRef CAS.
- F. Limé and K. Irgum, Macromolecules, 2009, 42, 4436–4442 CrossRef CAS; X. Z. Kong, X. L. Gu, X. Zhu and L. Zhang, Macromol. Rapid Commun., 2009, 30, 909–914 CrossRef CAS; G. Pan, B. Zu, X. Guo, Y. Zhang, C. Li and H. Zhang, Polymer, 2009, 50, 2819–2825 CrossRef CAS.
- A. Janošević, G. Ćirić-Marjanović, B. Marjanović, P. Holler, M. Trchoá and J. Stejskal, Nanotechnology, 2008, 19, 135606–135614 CrossRef.
Footnote |
| † General procedure for the cationic copolymerization of styrene and divinylbenzene: a 500 mL round bottom flask was charged with 250 mL of hexanes and the required amounts of ST (2.075 g) and DVB (2.075 g) were added (when using different percentages of ST and DVB the concentration of the solution was kept constant at 16.6 g of reactant per litre of solvent). The mixture was allowed to stir at 500 rpm at room temperature. Distilled grade boron trifluoride diethyl etherate initiator was added to the solution dropwise using a syringe over a period of 5 minutes. Typically, 5 parts of initiator per 100 parts of reactants has been used. Solid particle-like polymers suspended in the stirring solution appear during addition of the initiator. The resulting polymer particles are observed to grow in number with time. The reaction was terminated after 6 h by stopping the stirring, and the resulting materials were allowed to settle to the bottom of the flask and collected by filtration. The original yellow polymeric materials turned white upon washing with acetone. The isolated polymer was dried under vacuum for 12 h prior to analysis. |
| This journal is © The Royal Society of Chemistry 2010 |
