Peptide-coated, self-assembled M12L24 coordination spheres and their immobilization onto an inorganic surface†
Masatoshi
Ikemi
a,
Takashi
Kikuchi
a,
Sachiko
Matsumura
b,
Kiyotaka
Shiba
*b,
Sota
Sato
a and
Makoto
Fujita
*a
aDepartment of Applied Chemistry, School of Engineering, The University of Tokyo and CREST, Japan Science and Technology Agency (JST), 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan. E-mail: mfujita@appchem.t.u-tokyo.ac.jp; Fax: +81 3-5841-7257; Tel: +81 3-5841-7258
bDivision of Protein Engineering, Cancer Institute, Japanese Foundation for Cancer Research, 3-8-31 Ariake, Koto-ku, Tokyo 135-8550, Japan. E-mail: kshiba@jfcr.or.jp; Fax: +81 3-3570-0461; Tel: +81 3-3570-0489
First published on 21st May 2010
Abstract
A nano-sized M12L24 coordination sphere coated with 24 hexapeptide aptamers (Arg–Lys–Leu–Pro–Asp–Ala: minTBP-1) was efficiently self-assembled from 12 Pd(II) ions and 24 bent ligands with minTBP-1 at their convex. The high density of aptamers covering the 3.5 nm diameter sphere resulted in irreversible immobilization on a Ti surface, in contrast to the relatively weak, reversible binding of a single aptamer.
Efficient and site-selective immobilization of biomolecules such as proteins,1–6 viruses,7–11 and DNAs12–16 on inorganic surfaces is increasingly important in drug discovery,5,16 engineering biosensors,3,4,9,13–15 and the bottom-up fabrication of electronic devices.6,10–12 Peptide aptamers can noncovalently bind to specific inorganic surfaces and their application is promising due to high substrate specificity and the concomitant circumvention of surface pre-modification.17–21 The hexapeptide aptamer, minTBP-1 (Arg–Lys–Leu–Pro–Asp–Ala),21 specifically binds Ti surfaces through electrostatic interactions between the cationic Arg and Lys residues and the oxidized Ti surface in aqueous conditions. Attaching minTBP-1 to ferritin, the ubiquitous iron-storing protein, facilitates its adhesion onto a patterned Ti surface and has been used to fashion electronic devices.22–25
Self-assembled M12L24 sphere 2a (Fig. 1a) and its derivatives26–33 are easily modifiable and we have introduced many different functional groups into the sphere interior by simply attaching the desired moiety to the concave ligand. We thus envisioned that, by attaching minTBP-1 to the convex ligand edge, we could coat the periphery of sphere 2a with protein aptamers and enable the specific immobilization of well-defined functional nanoparticles on inorganic surfaces. And, even though the interaction of a single aptamer is relatively weak and reversible, we observed the irreversible binding of a self-assembled minTBP-1-coated M12L24 sphere to a Ti substrate, probably due to the dense coating of 24 peptide aptamers.
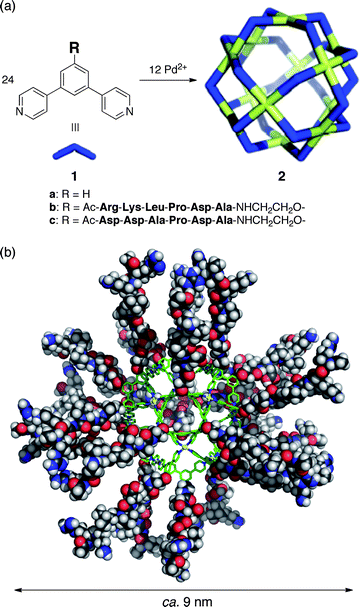 | ||
| Fig. 1 (a) Self-assembly of M12L24 spherical complexes 2a–c. (b) Molecular modelling of 2b. | ||
The minTBP-1 bearing ligand 1b was synthesized in 57% yield by coupling the protected aptamer 3 and the ligand precursor 4 using HBTU–HOBt–DIPEA followed by cleavage of the aptamer protecting groups with trifluoroacetic acid (Scheme 1 and ESI†). Ligand 1c, with anionic Asp residues replacing the cationic Arg and Lys (essential for binding to the Ti surface),21 was prepared in a similar manner for control experiments.
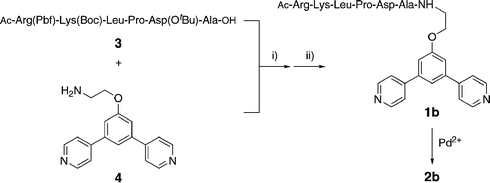 | ||
| Scheme 1 Preparation of ligand 1b and sphere 2b with pendant aptamers. (i) HBTU, HOBt, DIPEA; (ii) CF3COOH. | ||
The quantitative formation of M12L24 spherical complex 2b was observed when ligand 1b (14.7 mg, 0.01 mmol) was treated with Pd(NO3)2 (1.61 mg, 7.0 × 10−3 mmol) in DMSO (1 mL) at 70 °C for 24 h, (Fig. 2a, b).34 The notable downfield shifts of the signals of the pyridine α and β protons (Δδ = 0.76 and 0.36 ppm, respectively) are indicative of pyridyls coordinated to Pd(II) and the markedly broadened signals of a huge complex that moves slowly on the NMR time scale. A single band was observed by diffusion-ordered NMR spectroscopy (DOSY) with a diffusion coefficient of 3.8 ± 0.3 × 10−11 m2 s−1. From this diffusion constant, the diameter of the rigid core of 2b was calculated to be 3.9 ± 0.3 nm, consistent with the diameter estimated from molecular modeling.35 All the NMR (1D and DOSY) data are consistent with previous M12L24 spheres.26–33 Sphere 2c, prepared from ligand 1c, displayed similar behavior and properties.
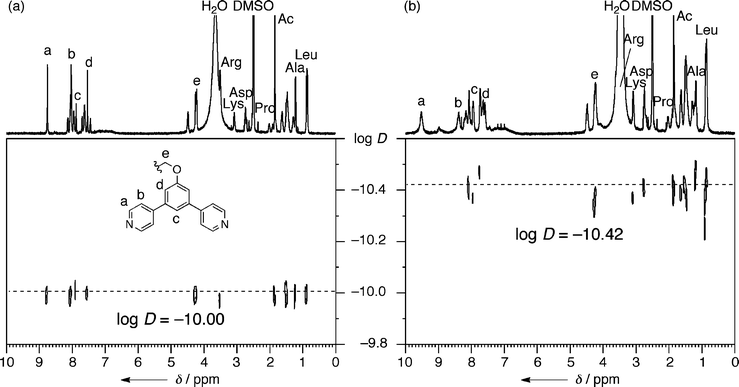 | ||
| Fig. 2 1H DOSY NMR spectra of (a) 1b and (b) 2b (500 MHz, DMSO-d6, 300 K). | ||
The optimized structure of sphere 2b revealed the 3.5 nm core framework coated with 24 minTBP-1 aptamers extending out for a total diameter of 8.8 nm (Fig. 1b). The surface density of minTBP-1 aptamers on 2b was calculated as 0.62 chains nm−2.
The high-density of clustered peptide aptamers should facilitate multipoint interactions and result in strongly immobilized spheres on a Ti surface. A single crystal TiO2 plate was immersed in an aqueous solution of 2b (1 μM) for 5 min and solution phase atomic force microscopy (AFM) revealed uniform spherical particles fully covering the surface (Fig. 3 and ESI†). The particle heights average at <4.4 nm, indicating that the peptide chains adopt folded conformations rather than being fully extended (8.8 nm). The spheres were strongly bound and even after repeated and thorough aqueous washes, the dissociation of spherical particles from the surface was not observed.
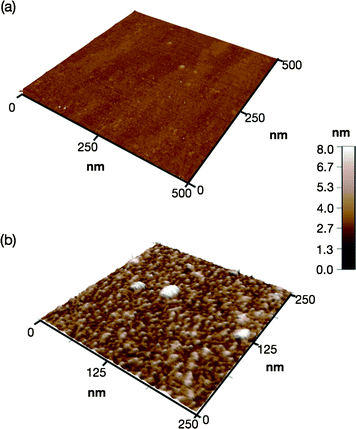 | ||
| Fig. 3 (a) Solution phase AFM images of a TiO2 surface (a) before and (b) after immobilization of 2b. | ||
Irreversible binding was further illustrated using quartz crystal microbalance (QCM) measurements with a Ti-coated sensor. When a diluted aqueous solution of 2b (10 μM, H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO = 95
DMSO = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (v/v)) buffered with 9.5 mM HEPES (pH 7.5) was injected into the QCM cell, the resonance frequency (f) of the Ti-coated sensor immediately decreased as 2b was bound to the Ti surface (Fig. 4, red line). The spheres were strongly bound and even after repeated washings with a buffer solution the f value remained constant. Using Sauerbrey's equation,36 the mass gain on the Ti sensor was estimated to be 3.7 × 102 ng cm−2 from Δf (−63 Hz). This mass gain, as well as the AFM observation, suggests that the sphere 2b was densely glued with the peptide aptamers on the Ti substrate in a monolayer fashion.
5 (v/v)) buffered with 9.5 mM HEPES (pH 7.5) was injected into the QCM cell, the resonance frequency (f) of the Ti-coated sensor immediately decreased as 2b was bound to the Ti surface (Fig. 4, red line). The spheres were strongly bound and even after repeated washings with a buffer solution the f value remained constant. Using Sauerbrey's equation,36 the mass gain on the Ti sensor was estimated to be 3.7 × 102 ng cm−2 from Δf (−63 Hz). This mass gain, as well as the AFM observation, suggests that the sphere 2b was densely glued with the peptide aptamers on the Ti substrate in a monolayer fashion.
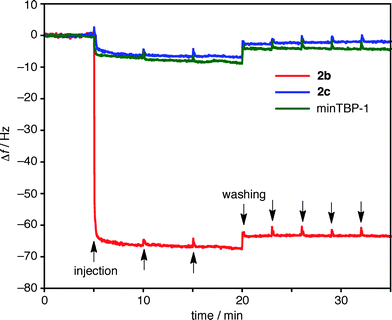 | ||
Fig. 4 Changes in resonance frequency (f) of a Ti sensor as a function of time in QCM measurements. Binding and dissociation profiles for 2b (red line), 2c (blue line), and minTBP-1 (green line) (H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO = 95 DMSO = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (v/v), 9.5 mM HEPES, pH 7.5, RT). 5 (v/v), 9.5 mM HEPES, pH 7.5, RT). | ||
The irreversible adhesion of the sphere on the Ti surface warrants further discussion. The interaction of a single aptamer on the Ti surface is quite weak: an injection of a solution of minTBP-1 under identical conditions caused only a minor change in resonance frequency (Fig. 4, green line). Bio-scaffolds, such as ferritin, with appended aptamers can show strong adhesion to inorganic surfaces but the aptamer densities are not particularly high. As a result, the binding is reversible and slow dissociation from the surface is inevitable. In contrast, the adhesion of 2b on a Ti surface is irreversible (Fig. 4), presumably due to efficient multipoint interactions from the dense display of aptamers coating the sphere periphery.
Sphere 2c, coated with 24 anionic peptides, was examined for comparison. Unlike minTBP-1, the anionic aptamer displays no strong association with Ti surfaces and, as a result, sphere 2c also does not bind to the Ti substrate surface. This result emphasizes that the specificity of the minTBP-1 sequence is essential for binding to the Ti surface and that multiple specific interactions result in irreversible binding for sphere 2b.
In conclusion, we have achieved the irreversible immobilization of a M12L24 coordination nanosphere on a Ti surface by attaching 24 peptide aptamers (minTBP-1) on the sphere periphery. The highlights of this method are: (i) the simple construction of the sphere framework by metal-directed self-assembly; (ii) irreversible adhesion to a TiO2 surface under mild conditions (neutral aqueous conditions at near room temperature); and (iii) no requirements for pre-modification of the metal surface.
We envision that immobilized nanospheres will open a variety of new avenues and applications in surface chemistry. Functional inorganic and organic nanoparticles could be enclathrated within the hollow coordination spheres and then immobilized on various inorganic and organic surfaces by simply choosing the appropriate aptamers as a sphere coating. Alternatively, incorporation of biomolecule binding motifs (e.g., antibodies, saccharides, or vitamins) into the sphere cavity or periphery could find applications where the spheres serve as receptors or glue between a biological substrate and a test surface.
Acknowledgements
This work was supported by the JST, Strategic International Cooperation Program (SICP) and MEXT, Grants-in-Aid for Young Scientists (A) (21685007).Notes and references
- L. S. Wong, F. Khan and J. Micklefield, Chem. Rev., 2009, 109, 4025–4053 CrossRef CAS.
- P. Jonkheijm, D. Weinrich, H. Schroeder, C. M. Niemeyer and H. Waldmann, Angew. Chem., Int. Ed., 2008, 47, 9618–9647 CrossRef CAS.
- D. Weinrich, P. Jonkheijm, C. M. Niemeyer and H. Waldmann, Angew. Chem., Int. Ed., 2009, 48, 7744–7751 CrossRef CAS.
- H. Zhu and M. Snyder, Curr. Opin. Chem. Biol., 2003, 7, 55–63 CrossRef CAS.
- G. C. Terstappen, C. Schlupen, R. Raggiaschi and G. Gaviraghi, Nat. Rev. Drug Discovery, 2007, 6, 891–903 CrossRef CAS.
- R. A. McMillan, J. Howard, N. J. Zaluzec, H. K. Kagawa, R. Mogul, Y. F. Li, C. D. Paavola and J. D. Trent, J. Am. Chem. Soc., 2005, 127, 2800–2801 CrossRef CAS.
- J. C. Smith, K. B. Lee, Q. Wang, M. G. Finn, J. E. Johnson, M. Mrksich and C. A. Mirkin, Nano Lett., 2003, 3, 883–886 CrossRef CAS.
- R. A. Vega, D. Maspoch, K. Salaita and C. A. Mirkin, Angew. Chem., Int. Ed., 2005, 44, 6013–6015 CrossRef CAS.
- C. Mao, A. Liu and B. Cao, Angew. Chem., Int. Ed., 2009, 48, 6790–6810 CrossRef CAS.
- R. J. Tseng, C. Tsai, L. Ma, J. Ouyang, C. S. Ozkan and Y. Yang, Nat. Nanotechnol., 2006, 1, 72–77 CrossRef CAS.
- C. E. Flynn, S.-W. Lee, B. R. Peelle and A. M. Belcher, Acta Mater., 2003, 51, 5867–5880 CrossRef CAS.
- S.-W. Chung, D. S. Ginger, M. W. Morales, Z. Zhang, V. Chandrasekhar, M. A. Ratner and C. A. Mirkin, Small, 2005, 1, 64–69 CrossRef CAS.
- A. Sassolas, B. D. Leca-Bouvier and L. J. Blum, Chem. Rev., 2008, 108, 109–139 CrossRef CAS.
- Y.-W. C. Cao, R. Jin and C. A. Mirkin, Science, 2002, 297, 1536–1540 CrossRef CAS.
- R. J. Lipshutz, S. P. A. Fodor, T. R. Gingeras and D. J. Lockhart, Nat. Genet., 1999, 21, 20–24 CrossRef CAS.
- J. Ziauddin and D. M. Sabatini, Nature, 2001, 411, 107–110 CrossRef.
- U. Kriplani and B. K. Kay, Curr. Opin. Biotechnol., 2005, 16, 470–475 CrossRef CAS.
- B. R. Peelle, E. M. Krauland, K. D. Wittrup and A. M. Belcher, Langmuir, 2005, 21, 6929–6933 CrossRef CAS.
- M. Sarikaya, C. Tamerler, A. K.-Y. Jen, K. Schulten and F. Baneyx, Nat. Mater., 2003, 2, 577–585 CrossRef CAS.
- R. L. Willett, K. W. Baldwin, K. W. West and L. N. Pfeiffer, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 7817–7822 CrossRef CAS.
- K. Sano and K. Shiba, J. Am. Chem. Soc., 2003, 125, 14234–14235 CrossRef CAS.
- K.-I. Sano, K. Ajima, K. Iwahori, M. Yudasaka, S. Iijima, I. Yamashita and K. Shiba, Small, 2005, 1, 826–832 CrossRef CAS.
- I. Yamashita, H. Kirimura, M. Okuda, K. Nishio, K. Sano, K. Shiba, T. Hayashi, M. Hara and Y. Mishima, Small, 2006, 2, 1148–1152 CrossRef CAS.
- K. Sano, H. Sasaki and K. Shiba, J. Am. Chem. Soc., 2006, 128, 1717–1722 CrossRef CAS.
- K. Sano, S. Yoshii, I. Yamashita and K. Shiba, Nano Lett., 2007, 7, 3200–3202 CrossRef CAS.
- M. Tominaga, K. Suzuki, M. Kawano, T. Kusukawa, T. Ozeki, S. Sakamoto, K. Yamaguchi and M. Fujita, Angew. Chem., Int. Ed., 2004, 43, 5621–5625 CrossRef CAS.
- M. Tominaga, K. Suzuki, T. Murase and M. Fujita, J. Am. Chem. Soc., 2005, 127, 11950–11951 CrossRef CAS.
- S. Sato, J. Iida, K. Suzuki, M. Kawano, T. Ozeki and M. Fujita, Science, 2006, 313, 1273–1276 CrossRef CAS.
- K. Suzuki, J. Iida, S. Sato, M. Kawano and M. Fujita, Angew. Chem., Int. Ed., 2008, 47, 5780–5782 CrossRef CAS.
- K. Suzuki, S. Sato and M. Fujita, Nat. Chem., 2010, 2, 25–29 Search PubMed.
- T. Kikuchi, T. Murase, S. Sato and M. Fujita, Supramol. Chem., 2008, 20, 81–94 CrossRef CAS.
- N. Kamiya, M. Tominaga, S. Sato and M. Fujita, J. Am. Chem. Soc., 2007, 129, 3816–3817 CrossRef CAS.
- T. Murase, S. Sato and M. Fujita, Angew. Chem., Int. Ed., 2007, 46, 5133–5136 CrossRef CAS.
- Although the ideal ratio of 1b
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd(NO3)2 is 2
Pd(NO3)2 is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Pd(II) ions are partially complexed with peptide chains and thus an excess of Pd(NO3)2 was required to complete the self-assembly into 2b.
1, Pd(II) ions are partially complexed with peptide chains and thus an excess of Pd(NO3)2 was required to complete the self-assembly into 2b. - The diameter of 2b was estimated from the Stokes–Einstein equation (standardized with X-ray structure of a relevant complex26).
- G. Sauerbrey, Z. Phys., 1959, 155, 206–222 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and characterization data. See DOI: 10.1039/c0sc00198h |
| This journal is © The Royal Society of Chemistry 2010 |
