Exploiting donor–acceptor interactions in aqueous dynamic combinatorial libraries: exploratory studies of simple systems†
Ho Yu
Au-Yeung
a,
Fabien B. L.
Cougnon
a,
Sijbren
Otto
b,
G. Dan
Pantoş
*a and
Jeremy K. M.
Sanders
*a
aUniversity Chemical Laboratory, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW, UK. E-mail: gdp26@cam.ac.uk; jkms@cam.ac.uk; Fax: +44 (0)1223 336017; Tel: +44 (0)1223 336411
bCentre for Systems Chemistry, Stratingh Institute, University of Groningen, Nijenborgh 4, 9747 AG, Groningen, The Netherlands
First published on 1st September 2010
Abstract
The behaviour of aqueous dynamic combinatorial libraries (DCLs) containing either electron-rich donor building blocks based on dioxynaphthalene (DN), or electron-deficient acceptor building blocks based on naphthalenediimide (NDI) are described. The influence of concentration and ionic strength on library distribution and diversity, together with the responses to electronically-complementary templates have been explored in detail, establishing the principles to be employed in more complex libraries leading to a new generation of catenanes.
Introduction
Dynamic combinatorial chemistry (DCC)1 has developed over the past decade from early proof-of-principle studies to the discovery of receptors,2 ligands,3 catalysts,4 sensors,5 and CO2-sequestering agents.6 In these systems, the evolution under thermodynamic control of the dynamic combinatorial library (DCL) is directed by intermolecular recognition between library members and an external component. Other DCLs include systems that take advantage of intermolecular interactions between all the species in solution, thereby addressing aggregation and self templation of library members.7 The exploitation of intramolecular interactions within library members leads to dynamic combinatorial approaches to the folding of proteins, nucleotides or synthetic polymers.8Despite these advances, systematic studies of dynamic libraries in general, and the discovery of unpredictable species from building blocks equipped with complementary recognition elements in particular, remain relatively unexplored. We elected to study donor–acceptor (DA) interactions between π-rich dioxynaphthalene (DN) donor units and π-deficient naphthalenediimide (NDI) acceptor units as a programmed stabilising inter- and intramolecular force in the context of DCC in water. DA components are usually aligned in parallel, with vivid colours often being observed due to charge transfer interactions. DA interactions are perhaps best described as a combination of local electrostatics, van der Waals and induction interactions, solvophobic effects and charge transfer processes. DA interactions have been successfully applied to create a wide range of supramolecular assemblies such as rotaxanes and catenanes,9 foldamers,10 molecular tweezers,11 synthetic channels and pores,12 organogelators13 and supramolecular polymers.14
We describe here an exploratory study of the DCL behaviour of three NDI and two DN dithiol building blocks (Fig. 1), including DCL formation, library diversity and templating effects. This paper focuses on DCLs that contain only either donor or acceptor building blocks as the reactive species. It provides the experimental basis and conceptual framework for our later discoveries of a whole series of new catenanes from aqueous DCLs.9f,g,o More broadly, this work is an entry point for the use of DA and hydrophobic interactions as intra- and/or inter-molecular drivers for the evolution of DCLs. Some of the results described here have previously been reported in preliminary form.2a
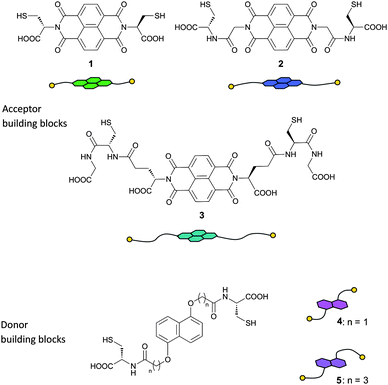 | ||
| Fig. 1 Structures of NDI acceptor (1–3) and DN donor (4 and 5) dithiol building blocks and their cartoon representations. | ||
Results and discussion
Building block designs and synthesis
All five building blocks follow the same general design: a central flat hydrophobic core which is electron-rich or electron-deficient, attached to two cysteine-derived flexible hydrophilic side-chains. NDI and DN were chosen as the central π-deficient and π-rich cores that can engage in DA interactions, while the amino acid side-chains provide both the thiol, for reversible disulfide exchange, and carboxylate groups, for water solubility.Syntheses of NDI 12a and DN 59f have been previously reported. NDI building block 2 was synthesised from the corresponding glycine derivative (Fig. 2), while NDI 3 was prepared from 1,4,5,8-naphthalenedianhydride and glutathione. DN dithiol 4 was prepared by a similar method to 5 (Fig. 3). The three NDI and two DN derivatives differ in the length and flexibility of their side-chains, allowing us to explore the effect of these structural changes on the DCL properties.
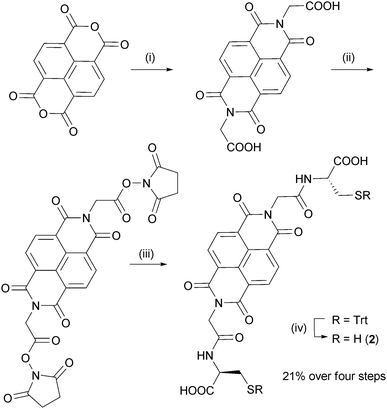 | ||
| Fig. 2 Synthesis of NDI dithiol 2. (i) Glycine, Et3N, DMF, 140 °C, μW, 5 min; (ii) N-hydroxysuccinimide, EDC·HCl, DMF, rt, 12 h; (iii) S-trityl-L-cysteine, Et3N, DMF, rt, 12 h; (iv) TFA, SiEt3H, rt, 2 h. | ||
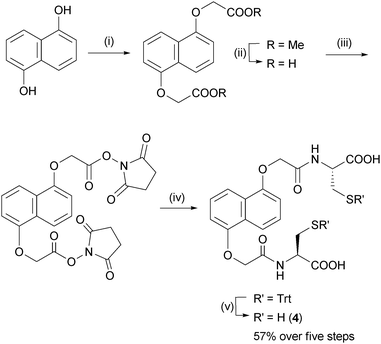 | ||
| Fig. 3 Synthesis of DN dithiol 4. (i) Methyl bromoacetate, K2CO3, acetone, reflux, 8 h; (ii) aq. NaOH/THF; (iii) N-hydroxysuccinimide, EDC·HCl, DMF, rt, 8 h; (iv) S-trityl-L-cysteine, Et3N, DMF, rt, 8 h; (v) TFA, SiEt3H, rt, 2 h. | ||
DCLs of the NDI acceptors
A first aqueous disulfide acceptor DCL was obtained by dissolving 1 in aq. NaOH (10 mM) to give a 5 mM solution, followed by titration with 0.1 M aq. NaOH to pH 8. The solution was stirred in a capped vial to allow atmospheric oxidation of the thiols. After five days, by which time equilibrium was reached, the DCL solution was analysed. HPLC and LCMS studies of the DCL solution revealed the presence of the cyclic dimer 6, cyclic trimer 7 and cyclic tetramer 8 in proportions of ca. 50%, 35% and 15% at equilibrium. Two separate peaks were observed for trimer 7 in the chromatogram, most likely due to two slowly interconverting conformers. All three cyclic library members (the major conformer for 7) were purified and further characterised by 1H NMR.2aDCL formation was monitored by analysing its composition at regular intervals (Fig. 4). No disulfide was detected in the first 15 min, but after one hour, the linear (6′) and cyclic (6) dimers were observed in significant quantities with some cyclic trimer 7. Cyclic tetramer 8 was first observed after two hours. During the first five hours, the concentration of 1 continuously decreased and that of the other cyclic oligomers increased, while the amount of the linear dimer 6′ remained more or less constant. These observations suggest that 6′ is the only major intermediate in the course of DCL development, being continuously formed and converted to cyclic species at similar rates during the first few hours. When the DCL was analysed after one day, conversion of 1 and 6′ to 6, 7 and 8 was completed. A DCL with similar distribution was obtained at day 2 and day 8, showing that thermodynamic equilibrium had been attained after one day.15
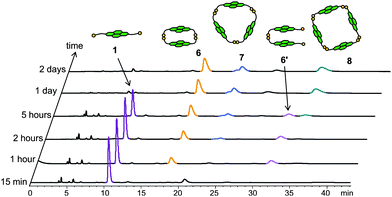 | ||
| Fig. 4 HPLC analysis of a 5 mM DCL of 1 at different time intervals. Absorbance was monitored at 383 nm. Peaks corresponding to different species are coloured accordingly. | ||
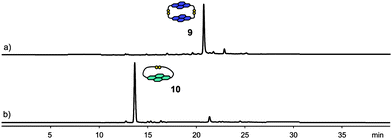
Aqueous disulfide DCLs of 2 and 3 were set up under similar conditions (5mM, pH 8). After equilibration for five days at room temperature, the two libraries consisted respectively of 9, the cyclic dimer of 2, and 10, the cyclic monomer of 3 (Fig. 5). The presence of a single product in both DCLs indicates that other oligomers, either linear or cyclic, are higher in energy. Therefore the potential surface describing the thermodynamic equilibrium of these libraries has one deep well in each case, corresponding to 9 and 10, respectively. The formation of 10 is not surprising, since the flexible side-chains of 3 are able to form an intramolecular disulfide bond, producing the entropically favourable cyclic monomer.
The libraries containing two or three NDI building blocks are expected to increase in complexity if the building blocks interact and form mixed species. This was indeed the case for the DCL containing 1 and 2, in which the mixed dimer 11 represents 45% of the library material. Homodimers 6 and 9 are present in 24% and 21%, while trimer 7 represents the remaining 10% of the library (Fig. 9a). In the DCL of 2 and 3 a mixed species, the heterodimer, was formed in ∼16% yield along with the homodimer of 2 and the cyclic monomer of 3 which form the rest of the library material (See ESI†). In contrast with this, the DCLs containing building blocks 1 and 3 did not show any significant mixing of the building blocks (See ESI†). This indicates that the mismatch in size between 1 and 3 leads to self-sorting of these building blocks; unsurprisingly the mixed DCL can be viewed as the sum of two independent libraries.
The most complex acceptor library containing all the NDI building blocks led to the formation of only one mixed species, heterodimer 11 (∼36% of the library material, Fig. 10a), along with macrocycles composed of only one type of NDI.
External stimuli: addition of guests
Since in this study we are interested in interactions between different π surfaces, a selection of aromatic molecules were tested as templates (Fig. 6). To enhance binding with the carboxylate-bearing anionic library members, only positively charged or neutral guests were used.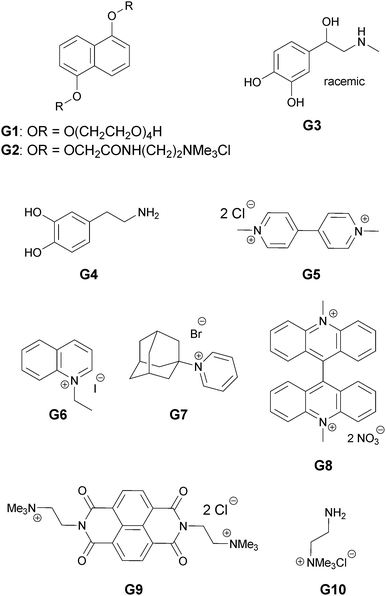 | ||
| Fig. 6 Guests G1–G10 tested in the DCLs. | ||
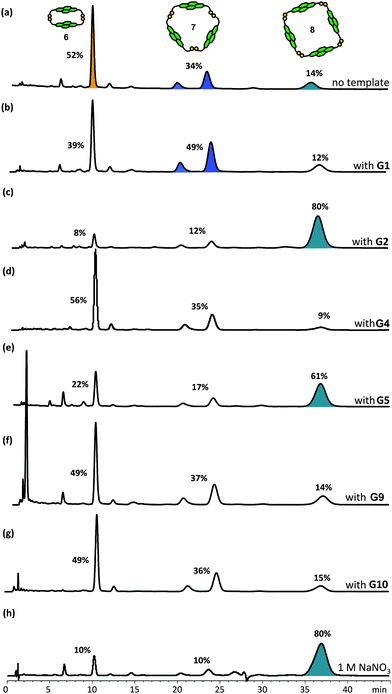 | ||
| Fig. 7 HPLC traces of DCLs of 5 mM of 1 (a) without template, and with 2.5 mM of (b) G1, (c) G2, (d) G4, (e) G5, (f) G9, (g) G10, and (h) with 1 M NaNO3. Absorbance was monitored at 383 nm. The DCL material distribution is represented as percentages above the corresponding peaks. The amplified species are highlighted. | ||
When the π donors G1 and G2 were added to the NDI 1 DCLs, an immediate colour change from yellow to plum (for G1) or orange (for G2) was observed, showing the presence of DA interactions between the NDI and DN units. Shifts in the equilibrium composition were also observed in these templated DCLs. For the neutral DN G1, an increase in the concentration of trimer 7 (both conformers) with a total amplification factor of 1.4 was observed. More pronounced amplifications were measured with the cationic DN G2: tetramer 8 was amplified up to 6-fold, corresponding to 80% of the DCL material (Fig. 7c). All the amplifications are at the expense of dimer 6. The continuing presence of 7 (both conformers) and the relative ratio of 6vs.7 (both conformers) in the DCLs templated by G2 indicate that trimers 7 are stabilised by binding G2, despite a reduction of their abundance in the library. A similar conclusion can be drawn from the library templated with G1 in which the presence of 8 and its abundance in the library indicate a stabilising interaction between this tetramer and the template. Dimer 6 has a cavity which is too small to accommodate any aromatic guests.2a
Despite the fact that G1 and G2 are derived from the same π-rich DN, different responses of the acceptor DCL were observed. Different macrocycles were amplified to different degrees, indicating that more than just DA interactions are involved in these recognition events. The modest amplification of trimer 7 by the neutral DN G1 and the strong amplification of tetramer 8 by cationic G2 indicate that DA interactions are relatively weak, and that the electrostatic interactions between the opposite charges are important for strong amplification.
The nature of the donor component is also important. Indeed, no significant change in the DCL distribution was observed for catechol-based neurotransmitters G3 and G4 although they are also π-rich and participate in DA interactions with the NDI to form deep olive coloured DCLs.16
On the other hand, despite their π-deficient nature, guests G5, G6 and G8 also amplified tetramer 8 from the DCL with amplification factors of 4.4, 2.1 and 2.1, respectively.17 No significant change in DCL distribution was observed for DCLs templated by G7, G9 or G10. Based on all these observations, we conclude that DA interactions are necessary but not sufficient to induce the large amplification of tetramer 8 as seen in the DCLs templated by G2. At the same time, the responses of the DCLs in the presence of G1, G2 and G4 also indicate that DA interactions alone are not sufficient to generate large amplifications. Hydrophobic and electrostatic interactions are also ruled out as being independently responsible for amplification of NDI macrocycles as G3, G4, G7, G9 and G10 have no influence on the equilibrium position of the DCL. Other factors such as the area of hydrophobic surface, steric effect and geometry of the templates may also be important but they are difficult to evaluate based on the current results.
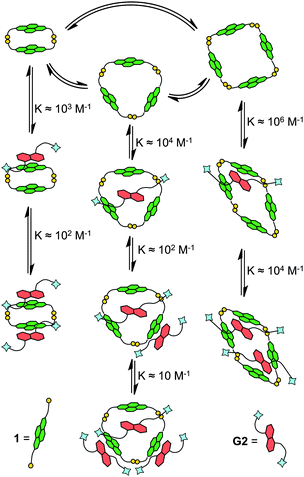 | ||
| Fig. 8 Model of DCL of 1 in the presence of G2 based on DCLFit and the respective binding constants obtained from the fitting. | ||
The interaction between the library members of a DCL of 1 with G2 was studied in detail using DCLFit software.18 This method relies on the HPLC peak areas obtained for each species present in the DCLs templated with various amounts of G2. The simplest model that produces a good fit requires the presence of a series of template-bound library members (Fig. 8). In the fitting model, all the association constants are for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes, using step-wise binding. The six-fold amplification of tetramer 8 by G2 is in accordance with the fitted binding constants of around 106 M−1 and 104 M−1 upon binding of the first and the second guest, respectively. The magnitude of these association constants suggests that there are two NDI moieties interacting with each of the DN templates. The association constant between one NDI and one DN is on the order of 103 M−1 as determined by DCLFit and supported UV-vis titrations.2a Trimer 7 binds one guest in its cavity (Ka ≈ 104 M−1). The cavity of dimer 6 is too small for an aromatic guest to fit inside, and therefore 6 binds to G2 using only the solvent-exposed surfaces of the NDIs.
1 complexes, using step-wise binding. The six-fold amplification of tetramer 8 by G2 is in accordance with the fitted binding constants of around 106 M−1 and 104 M−1 upon binding of the first and the second guest, respectively. The magnitude of these association constants suggests that there are two NDI moieties interacting with each of the DN templates. The association constant between one NDI and one DN is on the order of 103 M−1 as determined by DCLFit and supported UV-vis titrations.2a Trimer 7 binds one guest in its cavity (Ka ≈ 104 M−1). The cavity of dimer 6 is too small for an aromatic guest to fit inside, and therefore 6 binds to G2 using only the solvent-exposed surfaces of the NDIs.
In contrast with the DCL of 1, the libraries based on 2 or 3 were not influenced by the presence of any of the templates. This confirms that dimer 9 and cyclic monomer 10 are the most stable species in their respective libraries. This however does not exclude the possibility that electron rich templates like G1 and G2 or other aromatic guests can bind in the cavity of 9. This binding event would not lead to amplification of 9 since it is the only species formed in the untemplated library.
The untemplated DCLs containing two or three different NDI building blocks were more diverse and therefore more likely to lead to amplification of new species in the presence of templates. Indeed, in the library containing 1 and 2, G2 led to the amplification of heterodimer 11, with an amplification factor of 1.9 (Fig. 9). In this library, two G2 receptors containing exclusively NDI 1 could have been formed: trimer 7 and tetramer 8 (vide supra). Their absence signifies that the energetic gain from the formation of the G2·11 complex is larger than in the case of either G2·7 or G2·8. G2 was the only template that led to a change in the DCL distribution, highlighting again the importance of the π-rich aromatic core combined with cationic side-chains.
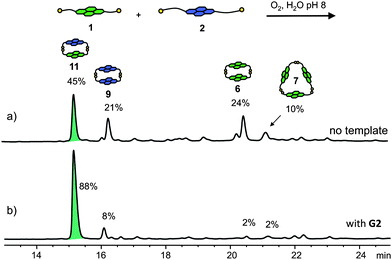 | ||
| Fig. 9 HPLC traces of a 5 mM DCL of 1 and 2 (a) without template and (b) with G2. Absorbance was monitored at 383 nm.22 | ||
The DCLs containing 1 and 3 were affected by the presence of templates G1 and G2. Trimer 7 was amplified by G1, while G2 led to the amplification of tetramer 8 and of a new heterodimer consisting of NDI 1 and 3 (amplification factor ∼ 30). These observations lead to the conclusion that only a strong binder like G2 induces the mixing of building blocks 1 and 3. In all other cases the two species do not interact, producing the same species as the ones observed in the DCLs containing each building block alone (See ESI†).
The DCL of 2 and 3 followed the pattern observed above, where addition of template G2 led to the amplification of the heterodimer of 2 and 3 (amplification factor 1.6), while all the other templates left the library distribution unchanged (See ESI†).
Unsurprisingly, the DCL containing all three NDI building blocks was perturbed by the presence of G2. In this templated DCL heterodimer 11 was amplified 2.3 fold at the expense of all other mixed macrocycles, indicating that the largest energetic gain is associated with the selective formation of the 11·G2 complex (Fig. 10).
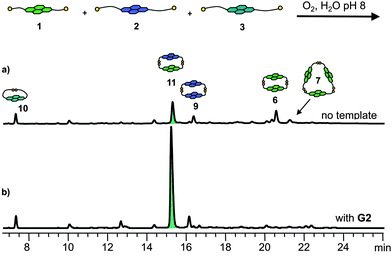 | ||
| Fig. 10 HPLC traces of the mixed DCLs of 5 mM (1.6 mM each) of 1, 2 and 3 (a) without template, and (b) with 2.5 mM of G2. Absorbance was monitored at 383 nm.22 | ||
External stimuli: increasing the solvent ionic strength
As the acceptor building blocks possess a large hydrophobic surface, the hydrophobic effect would be expected to have an important role in determining the stability and conformation of the various NDI macrocycles in water; it could therefore affect the equilibrium position of the DCL.19 A new set of DCLs under high ionic strength conditions (5 mM of NDI building block, 1 M NaNO3)20 were prepared and studied. In DCLs of 1, it was found that in these conditions, tetramer 8 was amplified to ca. 80% (Fig. 7h). This again showed that 8 is flexible enough to adopt a folded structure or aggregate to minimise the area of exposed hydrophobic surface under the high ionic strength. The libraries of 2 and 3 were not influenced by the ionic strength of the solution. No new species were formed under these conditions, highlighting again the stability of dimer 9 and cyclic monomer 10. Similar behaviour was observed for the mixed libraries containing 1 and 2, 1 and 3, and 2 and 3, respectively.All these observations allow us to conclude that the diversity of the DCLs is dependent on the very delicate balance between the length/flexibility of the sidearms and the rigidity of the NDI core. In this case a more rigid building block (1) gives a more diverse library than the more flexible 2 or 3, that can form two very stable species, dimer 9 and cyclic monomer 10. The mixed libraries lead to the formation of mixed NDI dimers whose formation was templated by π-rich, cationic template G2. The extent of the amplification factor for the homo- and heterodimers in the presence of this template might be the key for understanding the formation of DA catenanes via disulfide DCC.
DCLs of the DN donors
DCLs of donors 4 and 5 were prepared in an analogous way to the acceptor DCLs (5 mM of 4 or 5, pH 8). At equilibrium the DCL of 4 is composed of the cyclic monomer 12, the cyclic dimer 13 and the cyclic trimer 14 in proportions of 22%, 66% and 12%, respectively. In order to understand the DCL behaviour, its composition was monitored at relatively short time intervals for two days. The dithiol building block 4 was the only acyclic material detected during equilibration. All the three disulfide macrocycles were observed in the library just 15 min after set-up of the DCL, and their concentration increased as the DCL developed. Equilibrium was essentially reached after one day when no unreacted 4 was detected; the proportion of 12–14 remained the same after 2 days (Fig. 11).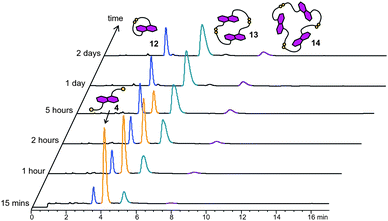 | ||
| Fig. 11 HPLC analysis of a 5 mM DCL of 4 at different time intervals. Absorbance was monitored at 292 nm. Peaks corresponding to different species are coloured accordingly. | ||
On the other hand, the DCL of 5 which possesses longer and more flexible side-chains is dominated at equilibrium by the cyclic monomer 15 (93%), with small amounts of the cyclic dimer 16 (6%) and the cyclic trimer 17 (<1%). Obviously, the longer and more flexible side-chains in 5 favour intramolecular disulfide formation, making 15—which is the most entropically favourable species—the major component in the DCL (see Fig. 12a and ESI†).
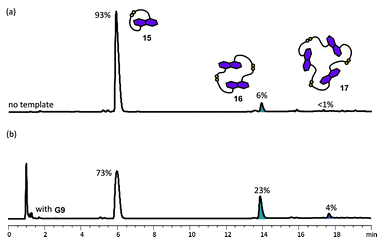 | ||
| Fig. 12 HPLC traces of DCLs of 5 mM of 5 (a) without template, and (b) with 2.5 mM of G9. Absorbance was monitored at 292 nm. The DCL material distribution is represented as percentages above the corresponding peaks. The amplified species are highlighted. | ||
As expected, the diversity of the DCL increases in a library containing both the donor building blocks. The library is dominated by the monomers (12 and 15) and dimers (13, 16 and 18), with some trimers observed (Fig. 13a). The observed ratio of dimers and trimers follows the same trend as expected for the flexibility of the macrocycles. The higher the rigidity of the dimer (and trimer), the higher its abundance in the library, even though the mixed-building blocks species are statistically more favourable.21 As in the case of the DCL of NDI 1 and 3, the size-mismatch between 4 and 5 leads to a decrease in concentration of the mixed species as a result of partial self-sorting.
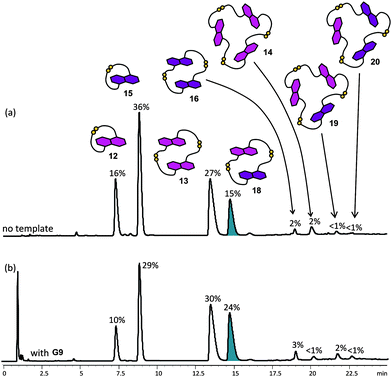 | ||
| Fig. 13 HPLC traces of the mixed DCLs of 5 mM (2.5 mM each) of 4 and 5 (a) without template, and (b) with 2.5 mM of G9. Absorbance was monitored at 292 nm. The DCL material distribution is represented as percentages above the corresponding peaks. The most amplified species is highlighted.22 | ||
External stimuli: addition of guests
The ability to influence the equilibrium distribution of the donor DCLs by π-deficient guests G5, G6, G7 and G9 was studied. Although colour changes due to DA interactions were observed upon addition of the acceptor guests to the donor DCL, no significant difference in the library composition of the DCL of the rigid donor 4 was found (See ESI†). Based on this, we conclude that cyclic dimer 13, which is the most probable host for forming a sandwich complex upon guest intercalation, is already the most stable and abundant species in the library, leaving very little room for further relative stabilisation. A similar argument was applied above to the NDI 2 DCLs templated by the electron-rich G1 and G2.In accordance with this view, amplification of the more flexible DN dimer 16, with an amplification factor of 3.8, was observed in a DCL of 5 templated by NDI guest G9 (5 mM of building block 5, 2.5 mM of guest). The concentration of trimer 17 was also increased to ca. 4% (Fig. 12). The other π-deficient templates did not significantly alter the DCL distribution, indicating that out of the tested templates, NDI template G9 has the best π-surface/geometry/charge match with the DN macrocycles (See ESI†).
The effect of NDI guest G9 on a mixed donor DCL prepared from an equimolar mixture of 4 and 5 was also studied (5 mM total concentration of building blocks, 2.5 mM template). The most significant change in the templated DCL was the amplification of mixed dimer 18 from 15% to 24%, suggesting that 18 may be the most suitable host for G9 compared to all other possible hosts in the DCL (Fig. 13). Its amplification was mainly at the expense of cyclic monomers 12 and 15. Small amplifications of homodimeric 13 and 16 were also observed. G6 and G8 influenced to a lesser extent this mixed DCL; the observed amplification factors were below 1.4 for the dominant species in the library (See ESI†).
External stimuli: increasing the solvent ionic strength
While the acceptor templates do not induce a significant change in the equilibrium position of the DCL of rigid donor 4, a very different DCL was obtained under high ionic strength conditions. Cyclic tetramer 21, which was not detected in the native DCL, was observed in ca. 9% abundance in a 5 mM DCL of 4 in the presence of 1 M NaNO3 (Fig. 14). Trimer 14 was also amplified by around two-fold in the DCL with a high-salt content at the expense of monomer 12 and dimer 13. Presumably, by analogy with NDI tetramer 8, DN tetramer 21 can fold or aggregate into a compact structure in solution, reducing the total area of exposed hydrophobic surfaces in response to the increased ionic strength, thereby increasing its concentration and stability. However, there is likely to be more repulsion between electron rich DNs than in the NDI tetramer, leading to a less stable folded structure for 21. The higher flexibility of the disulfide straps in 21 also imposes a higher entropic cost in its formation when compared to 8. The result of these opposing effects leads to a DCL of 4 containing only 9% of 21vs. the 80% of 8 present in the corresponding DCL of 1.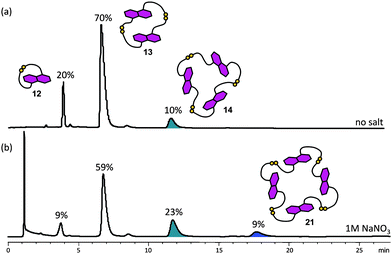 | ||
| Fig. 14 HPLC traces of DCLs of 5 mM of 4 (a) without salt, and (b) with 1 M of NaNO3. Absorbance was monitored at 292 nm. The DCL material distribution is represented as percentages above the corresponding peaks. Amplified species are highlighted.22 | ||
On the other hand, increasing solvent ionic strength by addition of NaNO3 to a DCL of 5 or the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixed DCL from 4 and 5 has no observable influence on the library distribution. (see ESI†). Presumably, this behaviour is due to the increased flexibility of 5 that leads to a higher entropic cost for the formation of higher oligomers that can fold better compared to the more rigid monomer and dimer. In these cases, in contrast to DCLs of 4, the entropic factors and electronic repulsions between the donor π-systems win over the hydrophobic interactions that lead to the formation of tetramer 21.
1 mixed DCL from 4 and 5 has no observable influence on the library distribution. (see ESI†). Presumably, this behaviour is due to the increased flexibility of 5 that leads to a higher entropic cost for the formation of higher oligomers that can fold better compared to the more rigid monomer and dimer. In these cases, in contrast to DCLs of 4, the entropic factors and electronic repulsions between the donor π-systems win over the hydrophobic interactions that lead to the formation of tetramer 21.
Conclusions
In summary, the DCL behaviours of DA building blocks that contain a central core of flat hydrophobic surface of NDI or DN and flexible hydrophilic thiol side-chains have been studied. The size and flexibility of building blocks have a crucial role in the type of products, DCL distributions and library response to templates.23 It has also been demonstrated that in addition to the use of electronically complementary guests, controlling hydrophobic effects through solvent ionic strength is an effective approach for perturbing the relative stability and manipulating the product distribution of the DA library members in these DCLs.This study further supports the idea expressed previously2a, 24 that building blocks which can be effectively incorporated into new receptors by templates in DCLs generally combine rigid and flexible subunits and incorporate recognition moieties within their structure; it is also apparent that large, flexible receptors are more responsive to guests and therefore bind more strongly. In addition we have shown that DCLFit can bring insights into otherwise intractable complex equilibria in DCLs.
This work has shown that DA interactions have been successfully engineered into DCC as a supramolecular handle to manipulate the DCL logically. It also provides a firm basis for studying how DA interactions direct the evolution of DCLs from building blocks installed with DA recognition units, providing an entry to the exploration of DCC in the area of inter-library members stabilisation and intramolecular folding.
Acknowledgements
We thank EPSRC, the Croucher Foundation and Pembroke College, Cambridge for financial support, and Dr Ana Belenguer for maintaining the HPLC facility.Notes and references
- For reviews, see: (a) A. Herrmann, Org. Biomol. Chem., 2009, 7, 3195 RSC; (b) S. Ladame, Org. Biomol. Chem., 2008, 6, 219 RSC; (c) J.-M. Lehn, Chem. Soc. Rev., 2007, 36, 151 RSC; (d) M. M. Rozenman, B. R. McNaughton and D. R. Liu, Curr. Opin. Chem. Biol., 2007, 11, 259 CrossRef CAS; (e) P. T. Corbett, J. Leclaire, L. Vial, K. R. West, J.-L. Wietor, J. K. M. Sanders and S. Otto, Chem. Rev., 2006, 106, 3652 CrossRef CAS; (f) B. de Bruin, P. Hauwert and J. N. H. Reek, Angew. Chem., Int. Ed., 2006, 45, 2660 CrossRef; (g) Dynamic Combinatorial Chemistry in Drug Discovery, Bioorganic Chemistry, and Materials Science, ed. B. L. Miller, Wiley, Hoboken, NJ., 2010 Search PubMed; (h) Dynamic Combinatorial Chemistry, ed. J. N. H. Reek and S. Otto, Wiley-VCH, Weinheim, 2010 Search PubMed.
- For recent examples: (a) H. Y. Au-Yeung, P. Pengo, G. D. Pantoş, S. Otto and J. K. M. Sanders, Chem. Commun., 2009, 419 RSC; (b) V. Saggiomo and U. Lüning, Chem. Commun., 2009, 3711 RSC; (c) L. A. Ingerman and M. L. Waters, J. Org. Chem., 2009, 74, 111 CrossRef CAS; (d) R. F. Ludlow and S. Otto, J. Am. Chem. Soc., 2008, 130, 12218 CrossRef CAS; (e) M.-K. Chung, C. M. Hebling, J. W. Jorgenson, K. Severin, S. J. Lee and M. R. Gagné, J. Am. Chem. Soc., 2008, 130, 11819 CrossRef CAS; (f) P. Besenius, P. A. G. Cormack, J. Liu, S. Otto, J. K. M. Sanders and D. C. Sherrington, Chem.–Eur. J., 2008, 14, 9006 CrossRef CAS; (g) S. M. Turega, C. Lorenz, J. W. Sadownik and D. Philp, Chem. Commun., 2008, 4076 RSC.
- For recent examples: (a) R. Caraballo, H. Dong, J. P. Ribeiro, J. Jiménez-Barbero and O. Ramström, Angew. Chem., Int. Ed., 2010, 49, 589; (b) D. E. Scott, G. J. Dawes, M. Ando, C. Abell and A. Ciulli, ChemBioChem, 2009, 10, 2772 CrossRef CAS; (c) R. Pérez-Fernández, M. Pittelkow, A. M. Belenguer, L. A. Lane, C. V. Robinson and J. K. M. Sanders, Chem. Commun., 2009, 3708 RSC; (d) G. Nasr, E. Petit, C. T. Supuran, J.-Y. Winum and M. Barboiu, Bioorg. Med. Chem. Lett., 2009, 19, 6014 CrossRef CAS; (e) P. C. Gareiss, K. Sobczak, B. R. McNaughton, P. B. Palde, C. A. Thornton and B. L. Miller, J. Am. Chem. Soc., 2008, 130, 16254 CrossRef CAS; (f) M. T. Cancilla, M. M. He, N. Viswanathan, R. L. Simmons, M. Taylor, A. D. Fung, K. Cao and D. A. Erlanson, Bioorg. Med. Chem. Lett., 2008, 18, 3978 CrossRef CAS; (g) M. C. Nielsen and T. Ulven, Chem.–Eur. J., 2008, 14, 9487 CrossRef CAS; (h) A. Bugaut, K. Jantos, J.-L. Wietor, R. Rodriguez, J. K. M. Sanders and S. Balasubramanian, Angew. Chem., Int. Ed., 2008, 47, 2677 CrossRef CAS.
- (a) G. Gasparini, L. J. Prins and P. Scrimin, Angew. Chem., Int. Ed., 2008, 47, 2475 CrossRef CAS; (b) B. Brisig, J. K. M. Sanders and S. Otto, Angew. Chem., Int. Ed., 2003, 42, 1270 CrossRef CAS.
- A. Buryak and K. Severin, Angew. Chem., Int. Ed., 2005, 44, 7935 CrossRef CAS.
- J. Leclaire, G. Husson, N. Devaux, V. Delorme, L. Charles, F. Ziarelli, P. Desbois, A. Chaumonnot, M. Jacquin, F. Fotiadu and G. Buono, J. Am. Chem. Soc., 2010, 132, 3582 CrossRef CAS.
- (a) A. G. Orrillo and R. L. E. Furlan, J. Org. Chem., 2010, 75, 211 CrossRef CAS; (b) R. A. R. Hunt, R. F. Ludlow and S. Otto, Org. Lett., 2009, 11, 5110 CrossRef CAS; (c) J. B. Zhang, B. W. Jing, N. Tokutake and S. L. Regen, J. Am. Chem. Soc., 2004, 126, 10856 CrossRef CAS; (d) J. M. A. Carnall, C. A. Waudby, A. M. Belenguer, M. C. A. Stuart, J. J.-P. Peyralans and S. Otto, Science, 2010, 327, 1502 CrossRef CAS.
- (a) Y. Krishnan-Ghosh, A. M. Whitney and S. Balasubramanian, Chem. Commun., 2005, 3068 RSC; (b) M. A. Case and G. L. McLendon, Acc. Chem. Res., 2004, 37, 754 CrossRef CAS; (c) Y. Krishnan-Ghosh and S. Balasubramanian, Angew. Chem., Int. Ed., 2003, 42, 2171 CrossRef CAS; (d) D. H. Zhao and J. S. Moore, J. Am. Chem. Soc., 2002, 124, 9996 CrossRef CAS; (e) M. Royo, M. A. Contreras, E. Giralt, F. Albericia and M. Pons, J. Am. Chem. Soc., 1998, 120, 6639 CrossRef CAS.
- For recent examples of DA rotaxanes and catenanes: (a) K. M. Mullen, J. J. Davis and P. D. Beer, New J. Chem., 2009, 33, 769 RSC; (b) T.-C. Lin, C.-C. Lai and S.-H. Chiu, Org. Lett., 2009, 11, 613 CrossRef CAS; (c) T. Ikeda, M. Higuchi and D. G. Kurth, Chem.–Eur. J., 2009, 15, 4906 CrossRef CAS; (d) I. Yoon, D. Benítez, Y.-L. Zhao, O.Š. Miljanić, S.-Y. Kim, E. Tkatchouk, K. C.-F. Leung, S. I. Khan, W. A. Goddard III and J. F. Stoddart, Chem.–Eur. J., 2009, 15, 1115 CAS; (e) A. M. Cagulada and D. G. Hamilton, J. Am. Chem. Soc., 2009, 131, 902 CrossRef CAS ; and catenanes:; (f) H. Y. Au-Yeung, G. D. Pantoş and J. K. M. Sanders, J. Am. Chem. Soc., 2009, 131, 16030 CrossRef CAS; (g) H. Y. Au-Yeung, G. D. Pantoş and J. K. M. Sanders, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 10466 CrossRef CAS; (h) V. Blanco, D. Abella, E. Pía, C. Platas-Iglesias, C. Peinador and J. M. Quintela, Inorg. Chem., 2009, 48, 4098 CrossRef CAS; (i) S. Li, M. Liu, B. Zheng, K. Zhu, F. Wang, N. Li, X.-L. Zhao and F. Huang, Org. Lett., 2009, 11, 3350 CrossRef CAS; (j) Y.-L. Zhao, A. Trabolsi and J. F. Stoddart, Chem. Commun., 2009, 4844 RSC; (k) J. F. Stoddart, Chem. Soc. Rev., 2009, 38, 1802 RSC; (l) J. F. Stoddart and H. M. Colquhoun, Tetrahedron, 2008, 64, 8231 CrossRef CAS; (m) W. R. Dichtel, O.Š. Miljanić, W. Zhang, J. M. Spruell, K. Patel, I. Aprahamian, J. R. Heath and J. F. Stoddart, Acc. Chem. Res., 2008, 41, 1750 CrossRef CAS; (n) L. Raehm, D. G. Hamilton and J. K. M. Sanders, Synlett., 2002, 1743 CAS; (o) H. Y. Au-Yeung, G. D. Pantoş and J. K. M. Sanders, Angew. Chem., Int. Ed., 2010, 49, 5331 CrossRef CAS.
- For examples: (a) W. Zhang, W. R. Dichtel, A. Z. Stieg, D. Benítez, J. K. Gimzewski, J. R. Heath and J. F. Stoddart, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 6514 CrossRef CAS; (b) V. J. Bradford and B. L. Iverson, J. Am. Chem. Soc., 2008, 130, 1517 CrossRef CAS; (c) S. Ghosh and S. Ramakrishnan, Angew. Chem., Int. Ed., 2004, 43, 3264 CrossRef CAS; (d) Q.-Z. Zhou, X.-K. Jiang, X.-B. Shao, G.-J. Chen, M.-X. Jia and Z.-T. Li, Org. Lett., 2003, 5, 1955 CrossRef CAS; (e) G. J. Gabriel and B. L. Iverson, J. Am. Chem. Soc., 2002, 124, 15174 CrossRef CAS.
- For examples: (a) A. Lohr, M. Grüne and F. Würthner, Chem.–Eur. J., 2009, 15, 3691 CrossRef CAS; (b) B. Branchi, V. Balzani, P. Ceroni, M. C. Kuchenbrandt, F.-G. Klärner, D. Bläser and R. Boese, J. Org. Chem., 2008, 73, 5839 CrossRef CAS; (c) H. M. Colquhoun, Z. Zhu, C. J. Cardin, Y. Gan and M. G. B. Drew, J. Am. Chem. Soc., 2007, 129, 16163 CrossRef CAS.
- For examples: (a) S. Hagihara, L. Gremaud, G. Bollot, J. Mareda and S. Matile, J. Am. Chem. Soc., 2008, 130, 4347 CrossRef CAS; (b) N. Sakai, A. L. Sisson, T. Bürgi and S. Matile, J. Am. Chem. Soc., 2007, 129, 15758 CrossRef CAS; (c) N. Sakai, A. L. Sisson, S. Bhosale, A. Fürstenberg, N. Banerji, E. Vauthey and S. Matile, Org. Biomol. Chem., 2007, 5, 2560 RSC; (d) H. Tanaka, G. Bollot, J. Mareda, S. Litvinchuk, D.-H. Tran, N. Sakai and S. Matile, Org. Biomol. Chem., 2007, 5, 1369 RSC.
- (a) P. Mukhopadhyay, Y. Iwashita, M. Shirakawa, S. Kawano, N. Fujita and S. Shinkai, Angew. Chem., Int. Ed., 2006, 45, 1592 CrossRef CAS; (b) B. G. Bag, G. C. Maity and S. K. Dinda, Org. Lett., 2006, 8, 5457 CrossRef CAS.
- (a) S. Fujii and J.-M. Lehn, Angew. Chem., Int. Ed., 2009, 48, 7635 CrossRef CAS; (b) F. Wang, B. Zheng, K. Zhu, Q. Zhou, C. Zhai, S. Li, N. Li and F. Huang, Chem. Commun., 2009, 4375 RSC.
- A re-equilibration study starting from an isolated sample of the cyclic tetramer 8 and 10 mol% of the reducing agent dithiothreitol led to the formation of the equilibrium mixture containing dimer 6, trimer 7 and tetramer 8.
- Reported pKa values of epinephrine G3 and dopamine G4 are 8.61–9.06, indicating that under the DCL conditions (pH 8) the protonated species represent more than 80% of the material present in the library. (a) A. E. Sánchez-Rivera, S. Corona-Avendaño, G. Alarcón-Angeles, A. Rojas-Hernández, M. T. Ramírez-Silva and M. A. Romero-Romo, Spectrochimica Acta Part A, 2003, 59, 3193 CrossRef CAS; (b) P. Y. Bruice, Organic Chemistry, 5th Ed., Person Prentice-Hall, New Jersey, 2007, 314 Search PubMed.
- Interactions between electron-deficient π-acceptors are also favourable due to a reduced repulsive interactions of the π systems, van der Waals interactions and hydrophobic effects: C. A. Hunter and J. K. M. Sanders, J. Am. Chem. Soc., 1990, 112, 5525 Search PubMed.
- R. F. Ludlow, J. Liu, H. Li, S. L. Roberts, J. K. M. Sanders and S. Otto, Angew. Chem., Int. Ed., 2007, 46, 5762 CrossRef CAS.
- For examples of the hydrophobic effect on dynamic systems: (a) M. Fujita, F. Ibukuro, H. Hagihara and K. Ogura, Nature, 1994, 367, 720 CrossRef CAS; (b) M. Fujita, F. Ibukuro and K. Ogura, J. Am. Chem. Soc., 1995, 117, 4175 CrossRef CAS.
- In this study we used NaNO3 to increase the solvent polarity. We have observed similar effects when using other inorganic salts such as KNO3, NaCl or Na2SO4.
- Cyclic monomer 12 is less favourable in the DCL than cyclic monomer 15 probably because of its higher ring strain. Indeed, monomer 12 is always present in a higher proportion than dimer 13 in the libraries.
- The absorbance of the library members depends on the number of the aromatic cores present in the molecule, irrespective of the length of the sidearms, i.e. at the same wavelength the value of the extinction coefficient of a dimer is expected to be twice that of the corresponding monomer.
- J. Liu, K. R. West, C. R. Bondy and J. K. M. Sanders, Org. Biomol. Chem., 2007, 5, 778 RSC.
- (a) P. T. Corbett, L. H. Tong, J. K. M. Sanders and S. Otto, J. Am. Chem. Soc., 2005, 127, 8902 CrossRef CAS; (b) J. K. M. Sanders, Chem.–Eur. J., 1998, 4, 1378 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed synthetic and library setup procedures, HPLC and LCMS methods and NMR spectra. See DOI: 10.1039/c0sc00307g |
| This journal is © The Royal Society of Chemistry 2010 |