Bow-tie metallo-cryptophanes from a carboxylate derived cavitand†‡
Tanya K.
Ronson
a,
Harriott
Nowell
b,
Aleema
Westcott
a and
Michaele J.
Hardie
*a
aSchool of Chemistry, University of Leeds, Leeds, UK. E-mail: m.j.hardie@leeds.ac.uk; Fax: +44 (0)113 343 6565; Tel: +44 (0)113 343 6458
bDiamond Light Source, Harwell Science and Innovation Campus, Didcot, UK
First published on 23rd June 2010
Abstract
A new carboxylic acid functionalised cavitand forms [Cu3L2] metallo-cryptophanes with Cu(OAc)2 that can be linked together into dimers with the bridging ligand 1,2-bis(4-pyridyl)ethylene. Reaction of the cavitand with Co(OAc)2 gives a metallo-cryptophane with a central Co7 cluster.
Cyclotriveratrylene (CTV) is a macrocyclic host molecule with a shallow molecular cavity and pyramidal shape.1 Derivatives of CTV and related cryptophanes have emerging applications in a range of areas including sensors and separations.2 A number of CTV analogues with appended ligand functionality capable of coordinating to transition metals are known,3–9 and some of these have been used to generate coordination polymers4–6 or discrete metallo-supramolecular assemblies.6–9 The majority of these larger assemblies employ pyridyl donors and contain single metal centres connected by tripodal ligands, although the catechol functionality of the demethylated derivative cyclotricatechylene (CTC) has also been utilised.9 The use of carboxylate ligands with appropriate metal centres introduces the possibility of utilising secondary binding units to construct metal–organic polyhedra and metal–organic frameworks, both of which have received considerable attention in recent years.10 While other carboxylic acid derived CTV analogues have been reported,5,11 the only example of their transition metal complexes is the Cu(II) 1-D coordination polymer with a carboxylate-decorated cryptophane reported by Mough and Holman.5
Cram et al.12 and Wytko and Weiss13 have demonstrated that the basic CTV host can be extended by bridging between the catechol units of CTC using a 1,3-substituted benzene (or similar) group. This has, however, remained a rare strategy for synthesising new CTV-based hosts. We have adopted this approach to target the carboxylate ligand tris[3,5-bis(methyl)benzoic acid]cyclotricatechylene (H3L) whose synthesis we report herein, along with its Cu(II) and Co(II) complexes.
Tris[3,5-bis(methyl)benzoic acid]cyclotricatechylene (H3L) was synthesised according to Scheme 1. The methyl ester Me3L was first prepared in 74% yield by the reaction of cyclotricatechylene with methyl-3,5-bis(bromomethyl)benzoate14 in dry degassed dimethylformamide (DMF) with Cs2CO3 as a base. The ligand H3L was obtained from the deprotection of Me3L with K2CO3 in MeOH/H2O followed by neutralisation with HCl.
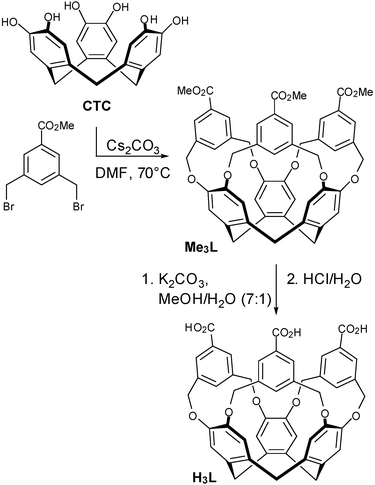 | ||
| Scheme 1 Synthesis of H3L. | ||
A 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of Cu(OAc)2 and H3L in dimethylformamide (DMF) was heated at 90 °C for 48 hours, then slowly cooled to room temperature to give greenish blue crystals of [Cu3L2(DMF)3]·2(DMF) 1 in 32% yield. Single crystal X-ray diffraction shows that the complex consists of a trimer of Cu(II) centres capped by two L3− ligands arranged in a head-to-head fashion, Fig. 1.§ The carboxylate groups each bridge two Cu(II) centres holding them in close proximity (Cu⋯Cu distances 3.50, 3.44, 3.46 Å). Each Cu(II) centre is coordinated by four carboxylate donors arranged in an approximate square plane and one DMF ligand occupying an axial position to give a square pyramidal geometry. The Cu–O(carboxylate) distances are in the range 1.9214(12)–1.9905(12) Å while the Cu–O(DMF) distances are longer at 2.4191(14), 2.3748(15) and 2.3115(14) Å. The ligand arms are directed inwards as a result of the metal-bridging behaviour of the carboxylates, rather than outwards as would be required to form more extended structures. This gives the metallo-cryptophane a pinched in and distinctive “bow-tie” appearance which is quite different from other examples of [M3L2] metallo-cryptophanes,8 or organic cryptophanes2 which have a larger guest accessible cavity in their central region.
1 mixture of Cu(OAc)2 and H3L in dimethylformamide (DMF) was heated at 90 °C for 48 hours, then slowly cooled to room temperature to give greenish blue crystals of [Cu3L2(DMF)3]·2(DMF) 1 in 32% yield. Single crystal X-ray diffraction shows that the complex consists of a trimer of Cu(II) centres capped by two L3− ligands arranged in a head-to-head fashion, Fig. 1.§ The carboxylate groups each bridge two Cu(II) centres holding them in close proximity (Cu⋯Cu distances 3.50, 3.44, 3.46 Å). Each Cu(II) centre is coordinated by four carboxylate donors arranged in an approximate square plane and one DMF ligand occupying an axial position to give a square pyramidal geometry. The Cu–O(carboxylate) distances are in the range 1.9214(12)–1.9905(12) Å while the Cu–O(DMF) distances are longer at 2.4191(14), 2.3748(15) and 2.3115(14) Å. The ligand arms are directed inwards as a result of the metal-bridging behaviour of the carboxylates, rather than outwards as would be required to form more extended structures. This gives the metallo-cryptophane a pinched in and distinctive “bow-tie” appearance which is quite different from other examples of [M3L2] metallo-cryptophanes,8 or organic cryptophanes2 which have a larger guest accessible cavity in their central region.
![The [Cu3L2(DMF)3] metallo-cryptophane from the crystal structure of complex 1. DMF ligands are shown in green.](/image/article/2011/CC/c0cc01284j/c0cc01284j-f1.gif) | ||
| Fig. 1 The [Cu3L2(DMF)3] metallo-cryptophane from the crystal structure of complex 1. DMF ligands are shown in green. | ||
The crystals of complex 1 were insoluble, so the solution properties of the complex could not be investigated. The solvent-free MALDI MS of the crystals shows a peak at m/z 1793.2 for the molecular ion species {Cu3(L)2H}+ with loss of the coordinated DMF ligands.
Similar reaction of Cu(OAc)2 and H3L in diethylformamide (DEF) gives the crystalline complex [Cu3L2(DEF)3]·(DEF) 2. The molecular structures of [Cu3L2(DMF)3] and [Cu3L2(DEF)3] have essentially the same [Cu3L2] cores, albeit with different axial ligands and show different crystal packing motifs (see ESI† for packing structure of 1 and the detailed structure of 2).
The axial DMF ligands of the [Cu3L2(DMF)3] metallo-cryptophane are weakly bound and it was anticipated that these could be displaced with linear bridging ligands in order to combine the [Cu3L2] units into larger clusters or networks. Hence, the reaction of H3L with Cu(OAc)2 was carried out in the presence of the potentially bridging ligands 1,2-bis(4-pyridyl)ethylene, 4,4′-bipyridine and tris-pyridyltriazine. The reaction with 1,2-bis(4-pyridyl)ethylene (BPE) gave very small blue crystals of complex [{Cu3L2(DMF)(H2O)}2(μ-BPE)]·4(DMF) 3 and their crystal structure was determined using synchrotron radiation. The complex consists of a discrete dimer of [Cu3L2(DMF)(H2O)] units linked together by bridging 1,2-bis(4-pyridyl)ethylene ligands, Fig. 2.
![Two views of the dimeric [{Cu3L2(DMF)(H2O)}2(μ-BPE)] assembly from the crystal structure of complex 3.](/image/article/2011/CC/c0cc01284j/c0cc01284j-f2.gif) | ||
| Fig. 2 Two views of the dimeric [{Cu3L2(DMF)(H2O)}2(μ-BPE)] assembly from the crystal structure of complex 3. | ||
The two [Cu3L2] metallo-cryptophane units in [{Cu3L2(DMF)(H2O)}2(μ-BPE)] are related to each other by a centre of inversion and are very similar to those of [Cu3L2(DMF)3] in complex 1 with two L3− ligands arranged around a Cu3 core in a head-to-head fashion. The 1,2-bis(4-pyridyl)ethylene ligand is coordinated axially to one of the Cu(II) centres of each [Cu3L2] unit at a Cu–N distance of 2.272(5) Å. It links two [Cu3L2] units in a linear fashion with a Cu⋯Cu separation of 13.85 Å. The remaining two Cu(II) centres of each [Cu3L2] unit are still coordinated by a terminal ligand (DMF or water). The structure is a dimer despite the presence of an excess of 1,2-bis(4-pyridyl)ethylene. Attempts to form more extended 2D networks with larger excesses of BPE did not give crystals suitable for X-ray analysis.
A 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of Co(OAc)2 and H3L in DMF was heated at 130 °C for 48 hours and slowly cooled to room temperature to give dark purple crystals of [Co7(μ3-L)2(μ3-OAc)4(μ4-O)2(DMF)2]·(DMF)·2(H2O) 4 along with some brown powder and oil. Single crystal X-ray diffraction shows that the complex consists of a cluster of seven Co(II) centres capped by two L3− ligands arranged in a head-to-head fashion, Fig. 3a.
1 mixture of Co(OAc)2 and H3L in DMF was heated at 130 °C for 48 hours and slowly cooled to room temperature to give dark purple crystals of [Co7(μ3-L)2(μ3-OAc)4(μ4-O)2(DMF)2]·(DMF)·2(H2O) 4 along with some brown powder and oil. Single crystal X-ray diffraction shows that the complex consists of a cluster of seven Co(II) centres capped by two L3− ligands arranged in a head-to-head fashion, Fig. 3a.
![Crystal structure of 4. (a) [Co7(μ3-L)2(μ3-OAc)4(μ4-O)2(DMF)2] with OAc in light blue and DMF in green; (b) ellipsoid plot of the bridged Co7 core with ellipsoids shown at 50% probability level. Symmetry operation i: −x, 2 − y, −z.](/image/article/2011/CC/c0cc01284j/c0cc01284j-f3.gif) | ||
| Fig. 3 Crystal structure of 4. (a) [Co7(μ3-L)2(μ3-OAc)4(μ4-O)2(DMF)2] with OAc in light blue and DMF in green; (b) ellipsoid plot of the bridged Co7 core with ellipsoids shown at 50% probability level. Symmetry operation i: −x, 2 − y, −z. | ||
The central Co7(μ-OAc)4(μ-O)2(DMF)2 cluster (Fig. 3b) is centrosymmetric and consists of two Co3 layers which are arranged staggered with respect to each other and a central Co centre [Co4 in Fig. 3b] which is not coordinated by any donor atoms from L3−. The cluster has four bridging acetate anions around the centre each of which bridge between Co(II) centres from the upper and lower Co3 layers and the central Co centre. The central Co centre is located on a centre of inversion and is coordinated by four bridging acetate anions and two oxide donors occupying the axial positions resulting in a distorted octahedral geometry. Co4–O bond lengths range from 2.010(4) to 2.190(5) Å, and the closest Co⋯Co contact is to Co1 at 2.8878(8) Å. Each ligand caps one of the Co3 layers with the carboxylate arms each bridging two Co(II) centres (Co⋯Co distances 3.310, 3.361, 3.229 Å). Each of these Co centres is only bound by carboxylate donors from one of the two L3− ligands rather than both as was observed for the Cu(II) centres in [Cu3L2(DMF)3]. The ligand arms are again bent inwards as a result of the bridging nature of the carboxylates. Co2 and Co3 have a tetrahedral geometry, coordinated by two carboxylate donors, one bridging acetate and one of the oxide centre (Co–O bond distances 1.930(4)–1.997(4) Å). Co1 has a distorted octahedral coordination environment and is bound by two carboxylate donors, two bridging acetates, one of the oxide centre and a DMF ligand and has slightly longer Co–O bond distances of 2.057(4)–2.236(5) Å. The metal cluster features a linear array of three face-sharing octahedra with vertex-sharing tetrahedra emanating the central octahedron. A number of Co7 clusters supported by organic ligands have been previously reported,15,16 however this motif has only been found in one other example—complex [Co7(μ4-O)2(μ3-OAc)4(μ2-OAc)6(OPEt3)2]—which also features the same pattern of bridging oxide and carboxylate ligands.16
In all cases, no resolved solvent or other guests were located in the hydrophobic interior cavity of the metallo-cryptophanes. Any residual electron density in these cavities was small (for example <1 e for complex 1). The volume of these cavities are ∼60 Å3 (complexes 1–3) and 70 Å for 4,17 which are too small to accommodate a molecule of DMF whose molecular volume is ∼77 Å3.
In summary, the new cavitand ligand H3L is a new member of an unusual and under-exploited class of CTV-based cavitands. The carboxylate moieties of L3− bridge between metal centres and promote the formation of high nuclearity M3 or M7 metal clusters to form novel metallo-cryptophanes. The “bow-tie” cryptophanes have pinched in, metalled cores, rather than large guest-accessible void space, and the [Cu3L2] complexes can be linked into a dimeric extended assemblyvia bridging ligands.
We thank EPSRC, STFC and the EPSRC Mass Spectrometry Service in Swansea for their support.
Notes and references
- A. Collet, Compr. Supramol. Chem., 1996, 6, 281 Search PubMed
.
- See recent reviews: T. Brotin and J.-P. Dutasta, Chem. Rev., 2009, 109, 88 Search PubMed
; M. J. Hardie, Chem. Soc. Rev., 2010, 39, 516 CrossRef CAS
.
- For example: D. Maiti, J. S. Woertink, R. A. Ghiladi, E. I. Solomon and K. D. Karlin, Inorg. Chem., 2009, 48, 8342 Search PubMed
; A. Gautier, J.-C. Mulatier, J. Crassous and J.-P. Dutasta, Org. Lett., 2005, 7, 1207 CrossRef CAS
; D. S. Bohle and D. J. Stasko, Inorg. Chem., 2000, 39, 5768 CrossRef CAS
; G. P. F. van Strijdonck, J. A. E. H. van Haare, J. G. M. van der Linden, J. J. Steggerda and R. J. M. Nolte, Inorg. Chem., 1994, 33, 999 CrossRef CAS
; G. Veriot, J.-P. Dutasta, G. Matouzenko and A. Collet, Tetrahedron, 1995, 51, 389 CrossRef
.
- C. Carruthers, J. Fisher, L. P. Harding and M. J. Hardie, Dalton Trans., 2010, 39, 355 RSC
; T. K. Ronson and M. J. Hardie, CrystEngComm, 2008, 10, 1731 RSC
.
- S. T. Mough and K. T. Holman, Chem. Commun., 2008, 1407 RSC
.
- C. Carruthers, T. K. Ronson, C. J. Sumby, A. Westcott, L. P. Harding, T. J. Prior, P. Rizkallah and M. J. Hardie, Chem.–Eur. J., 2008, 14, 10286 CrossRef CAS
.
- T. K. Ronson, J. Fisher, L. P. Harding, P. J. Rizkallah, J. E. Warren and M. J. Hardie, Nat. Chem., 2009, 1, 212 Search PubMed
; T. K. Ronson, J. Fisher, L. P. Harding and M. J. Hardie, Angew. Chem., Int. Ed., 2007, 46, 9086 CrossRef CAS
.
- A. Westcott, J. Fisher, L. P. Harding, P. Rizkallah and M. J. Hardie, J. Am. Chem. Soc., 2008, 130, 2950 CrossRef CAS
; Z. Zhong, A. Ikeda, S. Shinkai, S. Sakamoto and K. Yamaguchi, Org. Lett., 2001, 3, 1085 CrossRef CAS
.
- B. F. Abrahams, N. J. FitzGerald and R. Robson, Angew. Chem., Int. Ed., 2010, 49, 2896 CAS
.
- For recent reviews see: J. J. Perry, J. A. Perman and M. J. Zaworotko, Chem. Soc. Rev., 2009, 38, 1400 Search PubMed
; D. J. Tranchemontagne, J. L. Mendoza-Cortes, M. O'Keeffe and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1257 RSC
.
- For example: S. Le Gac, M. Luhmer, O. Reinaud and I. Jabin, Tetrahedron, 2007, 63, 10721 Search PubMed
; C. E. O. Roesky, E. Weber, T. Rambusch, H. Stephan, K. Gloe and M. Czugler, Chem.–Eur. J., 2003, 9, 1104 CrossRef CAS
; H.-Q. Zhan, X.-K. Jiang and Z.-T. Li, Chin. J. Chem., 2001, 19, 147 CrossRef CAS
.
- D. J. Cram, J. Weiss, R. C. Helgeson, C. B. Knobler, A. E. Dorigo and K. N. Houk, J. Chem. Soc., Chem. Commun., 1988, 407 RSC
.
- J. A. Wytko and J. Weiss, J. Inclusion Phenom. Mol. Recognit. Chem., 1994, 19, 207 CAS
; J. A. Wytko and J. Weiss, Tetrahedron Lett., 1991, 32, 7261 CrossRef CAS
.
- P. Liu, Y. Chen, J. Deng and Y. Tu, Synthesis, 2001, 2078 CrossRef CAS
.
- For example: L. F. Chibotaru, L. Ungur, C. Aronica, H. Elmoll, G. Pilet and D. Luneau, J. Am. Chem. Soc., 2008, 130, 12445 Search PubMed
; A. Ferguson, A. Parkin, J. Sanchez-Benitez, K. Kamenev, W. Wernsdorfer and M. Murrie, Chem. Commun., 2007, 3473 CrossRef CAS
; W.-Z. Wang, R. H. Ismayilov, G.-H. Lee, I. P.-C. Liu, C.-Y. Yeh and S.-M. Peng, Dalton Trans., 2007, 830 RSC
; Y.-Z. Zhang, W. Wernsdorfer, F. Pan, Z.-M. Wang and S. Gao, Chem. Commun., 2006, 3302 RSC
; E. K. Brechin, A. Graham, A. Parkin, S. Parsons, A. M. Seddon and R. E. P. Winpenny, J. Chem. Soc., Dalton Trans., 2000, 3242 RSC
.
- H. Ackermann, R. Leo, W. Massa and K. Dehnicke, Z. Naturforsch., B, 1998, 53, 1241 CAS
.
- Calculated using SQUEEZE: A. L. Spek, Acta Crystallogr., Sect. A, 1990, 46, C34 Search PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis and additional details on crystallographic studies and the structure of 2. CCDC 776456, 776458, and 776459. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0cc01284j |
| ‡ This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
§ Crystal data. Complex 1: C111H101Cu3N5O29, Mr = 2159.59, triclinic, a = 12.0641(11), b = 18.8982(17), c = 22.4670(19) Å, α = 94.765(4)°, β = 91.139(4)°, γ = 92.562(5)°, V = 5098.0(8) Å3, space groupP![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , Z = 2, θmax = 27.50°, 1366 parameters, R1 = 0.0335 (for 20056 data I > 2σ(I)), wR2 = 0.0938 (all data), S = 1.033. CCDC 776456. Complex 3: C111H94Cu3N4O28, Mr = 2122.52, triclinic, a = 17.140(4), b = 18.030(4), c = 20.805(5) Å, α = 86.235(12)°, β = 80.028(12)°, γ = 64.703(6)°, V = 5725(2) Å3, space groupP , Z = 2, θmax = 27.50°, 1366 parameters, R1 = 0.0335 (for 20056 data I > 2σ(I)), wR2 = 0.0938 (all data), S = 1.033. CCDC 776456. Complex 3: C111H94Cu3N4O28, Mr = 2122.52, triclinic, a = 17.140(4), b = 18.030(4), c = 20.805(5) Å, α = 86.235(12)°, β = 80.028(12)°, γ = 64.703(6)°, V = 5725(2) Å3, space groupP![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , Z = 2, θmax = 22.5°, 1291 parameters, R1 = 0.0808 (for 10527 data I > 2σ(I)), wR2 = 0.2289 (all data), S = 0.961. CCDC 776458. Complex 4: C113H103Co7N3O38, Mr = 2523.49, monoclinic, a = 18.811(3), b = 12.2580(15), c = 29.620(4) Å, β = 99.087(7)°, V = 6744.2(15) Å3, space groupP21/c, Z = 2, θmax = 25.00°, 745 parameters, R1 = 0.0805 (for 12651 data I > 2σ(I)), wR2 = 0.2401 (all data), S = 1.065. CCDC 776459. , Z = 2, θmax = 22.5°, 1291 parameters, R1 = 0.0808 (for 10527 data I > 2σ(I)), wR2 = 0.2289 (all data), S = 0.961. CCDC 776458. Complex 4: C113H103Co7N3O38, Mr = 2523.49, monoclinic, a = 18.811(3), b = 12.2580(15), c = 29.620(4) Å, β = 99.087(7)°, V = 6744.2(15) Å3, space groupP21/c, Z = 2, θmax = 25.00°, 745 parameters, R1 = 0.0805 (for 12651 data I > 2σ(I)), wR2 = 0.2401 (all data), S = 1.065. CCDC 776459. |
| This journal is © The Royal Society of Chemistry 2011 |
