Protein assembly along a supramolecular wire†‡
Marion K.
Müller
a,
Katja
Petkau
b and
Luc
Brunsveld
*ab
aChemical Genomics Centre of the Max Planck Society, Otto-Hahn Straße 15, 44227 Dortmund, Germany
bLaboratory of Chemical Biology, Department of Biomedical Engineering, Technische Universiteit Eindhoven, Den Dolech 2, 5612AZ, Eindhoven, the Netherlands. E-mail: l.brunsveld@tue.nl; Fax: +31 402478367; Tel: +31 402473737
First published on 23rd August 2010
Abstract
Discotic molecules self-assemble into supramolecular wires that act as platforms for directed protein assemblyvia appended biotin functionalities.
There is great interest in self-assembled nanostructures of proteins not only because protein assembly and aggregation play major roles in biological processes,1,2 also because, protein higher-order aggregates have found strong attention as novel materials for (bio)nanotechnology applications.3,4 Frequently, these protein assemblies have been obtained by using proteins or protein elements as building blocks.3–5 The utilization of synthetic structuring elements provides novel opportunities to direct and tune protein assembly characteristics such as stability and shape, either by furnishing the proteins with synthetic elements6–10 or with ordered synthetic frameworks that induce and control aggregation.11,12 Supramolecular polymers have emerged as attractive architectures in nanoscience in the fields of chemistry and biology,13,14 and allow access to additional larger protein assembly platforms.15 Novel dynamic supramolecular platforms are especially attractive because their reversible nature will allow reversible adaptivity in the protein assembly.14
Here we show how an auto-fluorescent, columnar supramolecular polymer can be used as self-assembling platform for protein assembly (Scheme 1). Discotic monomers reversibly assemble into a columnar polymer at low concentrations in water (1).16 By providing this self-assembled molecular wire with ligands (3) we now can utilize this system to assemble proteins. This is illustrated using biotin as a ligand that can attract either streptavidin or a biotin-antibody to the supramolecular platform. The supramolecular scaffold allows proteins to interact by inducing proximity along the wire.
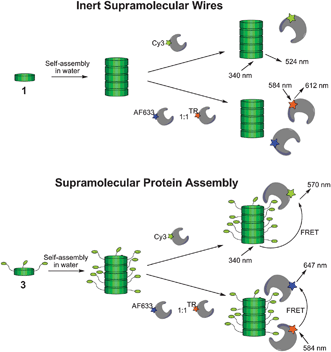 | ||
| Scheme 1 Discotic monomers decorated with ethylene glycols (1) self-assemble into inert supramolecular wires, devoid of interactions with proteins. Discotic monomer provided with biotin handles (3) assemble in columnar assemblies that induce peripheral protein assembly. Efficient supramolecular protein assembly is shown by energy transfer from the supramolecular platform to the proteins and in between the proteins. | ||
Discotic 1 (Scheme 2) self-assembles into highly fluorescent columnar structures at low concentrations in water.16 The decoration of these supramolecular polymers with ethylene glycol chains ensures good water-solubility and reduces unspecific interactions with biological matter.17 Based on discotic 1, first its analogue 2 with three peripheral amine functionalities was synthesized using a convergent multistep approach (see ESI‡). The peripheral amine groups were envisioned to enable rapid final stage modification of the discotic with ligands of interest. The introduction of the amine groups was based on selective mono-alkylation of gallic acid derivative 10 with a tosylated hexaethylene glycol furnished with a Boc protected amine. After hydrolysis of the gallic ester and acid chloride formation, the obtained molecule was coupled with 3,3′-diamino-2,2′-bipyridine. Subsequent reaction with benzene-1,3,5-tricarbonyl chloride led to a Boc protected discotic, which was deprotected to yield discotic 2. Subsequently in the final step, 2 was functionalized with biotin by threefold coupling with NHS-activated biotin in CH2Cl2 under ambient conditions to yield biotinylated 3. The threefold functionalization of the discotic proceeds rapidly in molecularly dissolved state, as non-assembled monomer, in CH2Cl2.
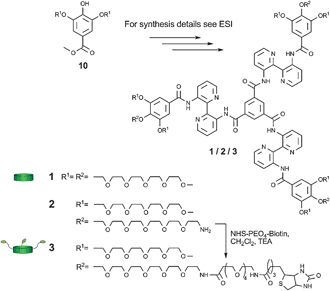 | ||
| Scheme 2 Chemical structures of discotics 1–3. | ||
Subsequently, the protein assembly of fluorescently labelled anti-biotin antibody (AB) and streptavidin (SA) proteins on supramolecular wires of 1 or of 3 was investigated. First, the assembly of Cy3® labelled streptavidin (Cy3-SA) and Cy3® labelled anti-biotin antibody (Cy3-AB) to biotinylated 3 aggregated in water was investigated using Förster resonance energy transfer (FRET). The dye Cy3® was selected as its absorbance spectrum overlaps with the emission spectrum of the discotic molecules 1 and 3. Protein assembly on the supramolecular wire is expected to induce close proximity of the Cy3® acceptor fluorophore and the discotic donor fluorophore. This would lead to a decrease in donor signal (emmax = 524 nm) with a simultaneous increase in acceptor signal (emmax = 570 nm). Non-biotinylated 1 was used as a control to study the interaction between the proteins and the discotics without any specific binding partners. Mixtures of supramolecular wires of 116 and the biotin binding proteins showed only the emission of the discotic donor (Fig. 1, black curve). Indeed, addition of Cy3-SA and of Cy3-AB to supramolecular wires formed by 3 resulted in a strong decrease in donor emission and the appearance of Cy3® acceptor emission (Fig. 1, red curve). This shows that the proteins are in close proximity to the columnar structure. This result shows that both Cy3-labelled streptavidin and Cy3-labelled anti-biotin antibody are capable of binding to the supramolecular wires of 3, and that this reaction is selectively induced by the appending biotins on the supramolecular scaffold. Also the results show a stronger energy transfer for the Cy3-SA than the Cy3-AB.
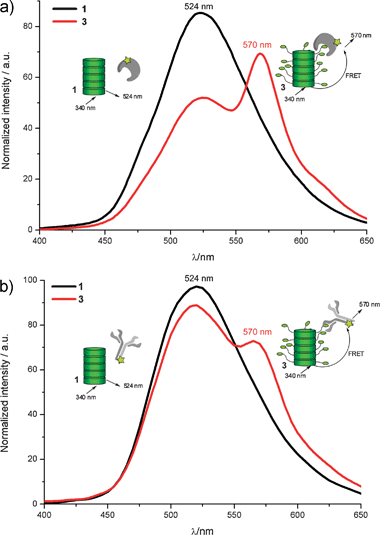 | ||
| Fig. 1 (a) Fluorescence spectra of Cy3® labelled streptavidin (0.02 μM) incubated with 1 (black) and 3 (red) (both at 1 μM). (b) Fluorescence spectra of Cy3® labelled anti biotin antibody (0.02 μM) with 1 (black) and 3 (red) (both at 1 μM). Spectra were recorded by using an excitation wavelength of 340 nm, and measured in phosphate buffer (pH 7.3). | ||
To investigate the protein assembly and the energy transfer process for both proteins in more detail, titration studies were performed (for titration curves see Fig. S1 and S2 in ESI‡). Different concentrations (from 0.3 nM to 1.5 μM) of either Cy3-SA or Cy3-AB were added to a 1 μM solution of 3. Upon addition of increasing amounts of Cy3-labelled protein, the fluorescence of the discotic donor decreased up to a complete quenching of donor fluorescence (Fig. 2). The titration curves clearly show that a higher concentration of Cy3-AB is required for efficient energy transfer than of Cy3-SA. For Cy3-AB, about half of the donor fluorescence is quenched after the addition of around 0.2 equivalents of Cy3-AB (0.2 μM in Fig. 2). For Cy3-SA only 0.02 equivalents suffice to quench half of the donor fluorescence (0.02 μM in Fig. 2). This implies that at this concentration the fluorescence of approximately 25 discotics in one supramolecular wire is quenched by one Cy3-SA acceptor fluorophore (donor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acceptor ratio is 50
acceptor ratio is 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and half of donor is quenched). Importantly, this result thus confirms16 the supramolecular assembly of the discotics in long molecular wires.
1 and half of donor is quenched). Importantly, this result thus confirms16 the supramolecular assembly of the discotics in long molecular wires.
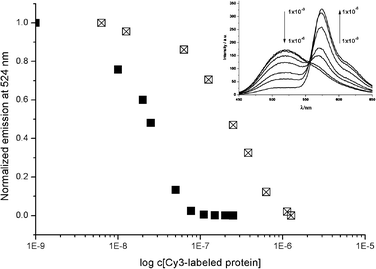 | ||
| Fig. 2 Normalized emission of the discotic donor (3, 1 μM) at 524 nm plotted against the concentration of Cy3® labelled protein (Cy3-SA (■) or Cy3-AB (⊠)). Inset: Fluorescence spectra of the Cy3-AB titration series. Spectra were recorded by using an excitation wavelength of 340 nm, and measured in phosphate buffer (pH 7.3). | ||
The differences in the FRET effects observed for the two types of proteins could result from several factors. Firstly, the antibody has a lower binding affinity for biotin (1.7 × 10−9 M)18 in comparison to streptavidin (4 × 10−14 M).19 Secondly, the number of biotin binding sites differs between the antibody (2) and streptavidin (4). Thirdly, the antibody (∼150 kDa) is three times as large as streptavidin (∼50 kDa). This final point can lead on the one hand to an increased distance between the dye and the discotic scaffold, and on the other hand to a steric crowding by the large antibody assembling on the supramolecular wire. Both effects would result in a lower FRET efficiency. The titration results thus show that the protein efficiently assembles on the supramolecular wire and that the protein characteristics direct the assembly process.
In order to demonstrate the ability of the supramolecular wire to act as a scaffold capable of inducing protein interactions, two different streptavidins labelled with a FRET pair (Alexa Fluor 633® and Texas Red®) were assembled on the supramolecular assemblies formed by 3. The biotin–streptavidin interaction should mediate the assembly of the streptavidins on the supramolecular wires and as such facilitate approximation of the FRET pair. This would lead to a decrease in TexasRed® emission (emmax = 612 nm) and an increase in Alexa Fluor 633® emission (emmax = 647 nm). Alexa Fluor 633-labelled streptavidin (A633-SA) and TexasRed-labelled streptavidin (TR-SA) were pre-incubated in a 1 to 1 ratio at a final concentration of 0.1 μM of each protein. Fluorescence spectra (excitation = 584 nm) were measured after the addition of 3, or of 1 as control at 1 μM. The emission spectrum of the FRET pair in the presence of assemblies formed by 1 (Fig. 3, black line) is similar to that without discotic (Fig. S3, ESI‡). However in the presence of supramolecular assemblies of 3, the donor signal of SA-TR almost disappears and the acceptor fluorescence of SA-AF633 significantly increases (Fig. 3, red curve). Even though the proteins are present at significantly lower concentration than the discotic (0.1 vs. 1 μM), an almost complete quenching of the SA-TR fluorescence is induced. This effect can only result from the recognition and assembly of the different streptavidins on the same supramolecular platform. This protein assembly induces the proximity of two fluorescently labelled proteins and enables non-radiative energy transfer from SA-TR to SA-AF633.
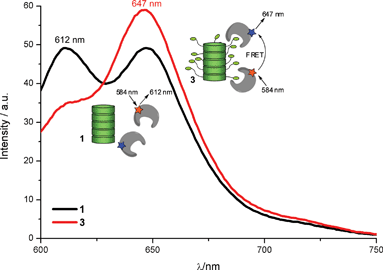 | ||
Fig. 3
Fluorescence spectra of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of TexasRed and Alexa Fluor 633 labelled streptavidins (each 0.1 μM) incubated with 1 (black) and 3 (red) (both at 1 μM). Spectra were recorded by using an excitation wavelength of 584 nm, and measured in phosphate buffer (pH 7.3). 1 mixture of TexasRed and Alexa Fluor 633 labelled streptavidins (each 0.1 μM) incubated with 1 (black) and 3 (red) (both at 1 μM). Spectra were recorded by using an excitation wavelength of 584 nm, and measured in phosphate buffer (pH 7.3). | ||
In conclusion, supramolecular polymeric wires based on discotic molecules provide attractive architectures for protein assembly. The supramolecular materials provide access to novel framework shapes, such as the columnar wires shown here, on which proteins can be made to aggregate and interact. On the supramolecular wire the proteins are brought to assemble via a specific interaction with biotin ligands displayed on the periphery. The approach shown here could also be used to assemble differently labelled proteins and make them interact as observed via inter-protein FRET.
The supramolecular approach is especially attractive as this now opens the possibility to attract different types of proteins to the scaffold simultaneously by utilizing mixtures of dynamically assembling discotics decorated with a variety of protein ligands. The supramolecular nature of the scaffold will allow for easy access to different assembly compositions due to the exchange of discotics within the wires,14,17 providing this system with unique properties to tune protein assembly and as functional material for energy transfer applications in chemical and materials sciences. Additionally, this supramolecular system might show potential as functional material at the single-molecule level.
The research was supported via a Sofja Kovalevskaja Award of the Alexander von Humboldt Foundation and the BMBF to LB.
Notes and references
- L. A. Lee, Z. W. Niu and Q. Wang Q, Nano Res., 2009, 2, 349–364 Search PubMed.
- T. Douglas and M. Young, Science, 2006, 312, 873–875 CrossRef CAS.
- T. Scheibel, Curr. Opin. Biotechnol., 2005, 16, 427–433 CrossRef CAS.
- M. G. Ryadnov, A. Bella, S. Timson and D. N. Woolfson, J. Am. Chem. Soc., 2009, 131, 13240–13241 CrossRef CAS.
- J. E. Padilla, C. Colovos and T. O. Yeates, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 2217–2221 CrossRef CAS.
- G. A. Silva, C. Czeisler, K. L. Niece, E. Beniash, D. A. Harrington, J. A. Kessler and S. I. Stupp, Science, 2004, 303, 1352–1355 CrossRef CAS.
- T.-F. Chou, C. So, B. R. White, J. C. T. Carlson, M. Sarikaya and C. R. Wagner, ACS Nano, 2008, 2, 2519–2525 CrossRef CAS.
- K. Matsuura, K. Murasato and N. Kimizuka, J. Am. Chem. Soc., 2005, 127, 10148–10149 CrossRef CAS.
- H. Kitagishi, Y. Kakikura, H. Yamaguchi, K. Oohora, A. Harada and T. Hayashi, Angew. Chem., Int. Ed., 2009, 48, 1271–1274 CrossRef CAS.
- S. Burazerovic, J. Gradinaru, J. Pierron and T. R. Ward, Angew. Chem., Int. Ed., 2007, 46, 5510–5514 CrossRef CAS.
- M. A. Kostiainen, O. Kasyutich, J. J. L. M. Cornelissen and R. J. M. Nolte, Nat. Chem., 2010, 2, 394–399 Search PubMed.
- E. H. M. Lempens, I. van Baal, J. L. J. van Dongen, T. M. Hackeng, M. Merkx and E. W. Meijer, Chem.–Eur. J., 2009, 15, 8760–8767 CrossRef CAS.
- J.-M. Lehn, Chem. Soc. Rev., 2007, 36, 151–160 RSC.
- D. A. Uhlenheuer, K. Petkau and L. Brunsveld, Chem. Soc. Rev., 2010, 39, 2817–2826 RSC.
- M. Felici, M. Marza-Perez, N. S. Hatzakis, R. J. M. Nolte and M. C. Feiters, Chem.–Eur. J., 2008, 14, 9914–9920 CrossRef CAS.
- L. Brunsveld, B. G. G. Lohmeijer, J. A. J. M. Vekemans and E. W. Meijer, Chem. Commun., 2000, 2305–2306 RSC.
- M. K. Müller and L. Brunsveld, Angew. Chem., Int. Ed., 2009, 48, 2921–2924 CrossRef CAS.
- H. Jung, T. Yang, M. D. Lasagna, J. Shi, G. D. Reinhart and P. S. Cremer, Biophys. J., 2008, 94, 3094–3103 CrossRef CAS.
- N. M. Green, Methods Enzymol., 1990, 184, 51–67 CAS.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Synthesis protocols and spectroscopy protocols. See DOI: 10.1039/c0cc02084b |
| This journal is © The Royal Society of Chemistry 2011 |
