Controlled production of polymer microspheres from microgel-stabilized high internal phase emulsions†‡
Zifu
Li
,
Xiaoling
Wei
and
To
Ngai
*
Department of Chemistry, The Chinese University of Hong Kong, Shatin N.T., Hong Kong. E-mail: tongai@cuhk.edu.hk; Fax: (852) 2503 5057; Tel: (852) 2696 1222
First published on 23rd August 2010
Abstract
We describe a flexible method for large-scale production of polymeric microspheres by templating a microgel-stabilized oil-in-water (o/w) high internal phase emulsion.
Micrometre- and nanometre-sized spherical particles play important roles in many applications, including drug delivery,1 catalysis,2 optoelectronics,3,4 and as templates to fabricate advanced materials.5,6 Inorganic particles are usually prepared through the reduction of a metal salt, or the controlled mixing of salt solutions.7 Various ingenious techniques including micro-emulsion,8 mini-emulsion,9 emulsion,10 dispersion,11precipitation,12 suspension13 and inverse suspension14 polymerization are being developed to synthesize or fabricate polymer microspheres with varying shapes and sizes. In a typical emulsion polymerization,10 a monomer is emulsified in an aqueous solution containing a suitable surfactant and an initiator molecule. Upon heating this mixture, particles are first nucleated from surfactant micelles and then continue to grow in size until the desired diameter is reached. Recently, preparation of liquid-core polymer shell microcapsules,15,16 hollow microspheres17 and porous polymer microspheres18 using colloidal particle stabilized internal phase emulsions has also been reported. Another well-established method to prepare organic or polymeric particles is the so called precipitation and condensation, which has been comprehensively reviewed by Horn and Rieger.19,20 Here, a solution of the organic or polymeric materials dissolved in a suitable solvent is added into an excess of a poor or non-solvent. This leads to super-saturation of the solution, and the organic or polymeric material consequently precipitates by separation of the solvent by evaporation or diffusion procedures, forming a dispersion of particles. The process of particle formation can be controlled, by for example adding polymers or surfactants that act as a protective colloid. However, one main disadvantage of this simple method is that a dilute solution of organic material is added to a large excess of non-solvent, resulting in very dilute particle dispersion. Therefore, the development of a versatile method for the scalable preparation of polymer particle dispersions is highly desirable.
Here we report a flexible method for production of highly concentrated dispersions of various polymer microspheres by templating a microgel-stabilized high internal phase emulsion (HIPE). HIPEs are a special type of emulsion where the volume fraction of the dispersed phase exceeds 0.74, the threshold value for monodisperse hard spheres.21 They occur as end products in a wide range of areas including the food, cosmetic, pharmaceutical and petroleum industries.22 Conventionally, polymerization reactions were performed to polymerize either the internal phase or continuous phase monomers and thus to obtain concentrated polymer latexes,23 composite materials24 or even porous materials.25 Herein we first generate an oil-in-water (o/w) emulsion (the internal oil volume fraction is 0.8) using an organic solvent oil containing a dissolved polymer, and a continuous phase containing a stabilizer, microgel particles. As the oil is slowly removed, polymer precipitation takes place in the emulsion droplets and ultimately leads to solid microspheres. Because of the highly concentrated emulsion droplets, this approach allows large scale production of polymer microspheres without involving any chemical reactions. More importantly, the microsphere size is proscribed by the size of the emulsion droplet, and its morphology is governed by the volatility of the used organic solvents. The ease with which these microspheres can be fabricated and the ability to tailor their shapes should facilitate investigation of their scope for practical applications.
A schematic illustration of the three-step process used to produce polymer microspheres with various structures is shown in Fig. 1. The process was exemplified using a model water-insoluble polymer, polystyrene (PS) which was synthesized by anionic polymerization (Mw ∼ 1.9 × 105 g mol−1, Mw/Mn ∼ 1.06, ESI†). In step I, organic solvent (benzene, toluene or o-xylene) with the dissolved PS chains (5 wt%) is mixed turbulently with an aqueous phase. The aqueous phase contains synthesized poly(N-isopropylacrylamide-co-methacrylic acid) (PNIPAM-co-MAA) flexible microgel particles (ESI†) that act as protective colloids. The turbulent mixing directly leads to a stable o/w HIPE (o/w ratio 80/20, 2 wt% microgel particles in aqueous solution) even though containing PS chains dissolved within the oil phase (Fig. S3, ESI†). Previously, we have shown that emulsions with an internal oil volume fraction up to 0.9 can be efficiently stabilized by PNIPAM-based microgel particles alone, that is, in the absence of surfactants.26 This efficiency stems from the fact that the microgel particles are not only adsorbed at the oil–water interface to hinder droplet coalescence, the excess microgel particles in the aqueous phase are probably contiguous with those adsorbed, thus serving to bind the oil droplets together into a three-dimensional network, in turn trapping the oil droplets in the gel matrix.26,27 The formed gel-like emulsions are extremely stable and inhibit the gravity-induced separation. Confocal images (Fig. S3, ESI†) of perylene-loaded emulsions confirm the presence of polydispersed oil droplets, whose size ranges from several micrometres (in benzene) to tens of micrometres (in toluene and o-xylene) that will act as microcompartments to limit the size of microspheres formed therein.
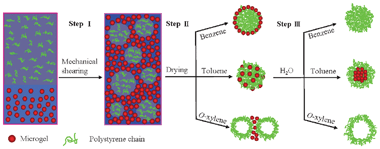 | ||
| Fig. 1 Schematic illustration of three-step process used to obtain microspheres with various structures by emulsion templating. | ||
The second step in the process was the elimination of the oil component and water by directly drying the gel-like emulsions in air (step II in Fig. 1). As the oil is slowly removed, the polymer concentrates in each emulsion droplet and ultimately solidifies. Meanwhile, the protective colloids, microgel particles which adsorbed at the oil–water interface and presented in the external continuous phase, form a solid support film upon removal of both water and organic solvent.14 The dried species were fully characterized by scanning electron microscope (SEM) and confocal microscope. It is interesting to note that depending on the used oil, the resultant structures are quite distinct. When using benzene as organic solvent, the SEM image in Fig. 2(a) shows that the PS microspheres prepared from emulsion templating have smooth surfaces and uniform spherical morphologies. The average diameter of the microspheres is 5 μm with a size distribution of 3 μm, which correlated well with the oil droplet diameters of the precursor emulsions (Fig. S3, ESI†), thus suggesting that the size of the resultant microsphere is proscribed by the size of the emulsion droplet. This is in agreement with the common features of solvent-based particle formation routes, described mainly as precipitation and condensation processes.17,18,28 Because the fluorescent microgel particles (red color) were used and perylene green dye was added to the oil phase, the dried samples were also visualized by confocal microscope. Note that for benzene as the organic solvent, the confocal images in Fig. 2(b) and (c) clearly revealed a core–shell like structure of the obtained microspheres with collapsed PS chains as the core and fluorescent microgel particles as the shell. In comparison, Fig. 2(e) and (f) showed that PS chains and microgel particles were randomly distributed and likely interlocked with each other to form microspheres on using toluene as the organic solvent. The SEM image (Fig. 2d) indicates that the size of the microspheres is slightly larger; the average diameter of the microspheres is 15 μm with a size distribution of 5 μm. However, it is quite remarkable to see that the replacement of organic solvent with o-xylene leads to a porous film instead of the microspheres (Fig. 2g–i). The average cavity sizes in the porous structures are comparable to the oil droplet diameters of the initial emulsions, thus indicating that these cavities result from a loss of the oil component. The retention of the macroporous structure suggests that using microgel well-stabilized emulsions as templates is sufficient to withstand the high capillary stresses developed during drying.
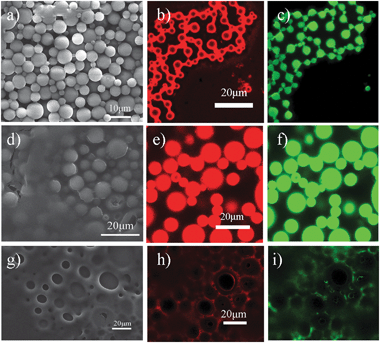 | ||
| Fig. 2 SEM images of microspheres or porous materials based upon the use of different organic solvents: (a) benzene, (d) toluene and (g) o-xylene after the removal of both oil and water at room temperature. Confocal images of dried samples: (b, c) core–shell type microspheres templating from benzene-in-water HIPEs; (e, f) microspheres templating from toluene-in-water HIPEs; (h, i) porous film templating from o-xylene-in-water HIPEs. Lasers with wavelengths 543 and 408 nm were used; fluorescent microgel particles, red; oil with dissolved perylene, green. | ||
Why is there so much difference in structure of the so-produced microspheres based upon the use of different organic solvents? To interpret the results shown above, the vapour pressure of the used organic solvents was first considered. At room temperature, the vapour pressure of benzene (13.3 kPa) is much higher than that of water (3.16 kPa). In this way, it is easy to understand that the precipitation and condensation of polymer chains inside the oil droplets is very fast during drying because of the high evaporation rate of benzene. The resultant microspheres thereby contain mainly collapsed PS chains as the core by absorbing a layer of interfacial microgel particles after evaporation of the water, as shown in Fig. 2(b). When the solvent is changed to toluene (3.65 kPa), which has a similar vapour pressure to water, the polymer shrinkage taking place by solvent evaporation inside the droplet occurs almost at the same rate to the precipitation of microgel particles at both the interfacial boundary and external continuous phase. It is likely that some of the interfacial microgel particles can freely diffuse into and interconnect with the collapsed PS chains to form the microspheres as schematically illustrated in step II of Fig. 1 and confirmed by Fig. 2(e). When a solvent with a much lower vapour pressure, for example, o-xylene (0.93 kPa) is used, the solvent evaporation in the continuous aqueous phase is expected to be faster. This may lead to a polymer gradient inside the emulsion droplets, namely, the PS concentration at the edge of the droplets would increase quicker and first precipitate due to the faster solvent evaporation in comparison with that of the inner part of the droplets. As a result, porous structure with a layer of polymer deposited at the edge is obtained as shown in Fig. 2(g–i), which will be further verified in the next section.
The final step in the process was to remove the formed microgel supporting film and disperse the produced microspheres (step III in Fig. 1). This was done by immersing the corresponding dried species in water for two days. For microspheres produced from organic solvent benzene, Fig. 3(a) shows that the initial core–shell structure microspheres are turned to solid microspheres, indicating that the adsorbed microgel particles in the shell are washed away. The microspheres are spherical and have a smooth surface as seen in Fig. 3(b). It is surprising to see that in the case of toluene as organic solvent, core–shell microspheres are produced after immersing in water (Fig. 3c). The majority of the microspheres have a diameter in the range of 5–15 μm (Fig. 3d). The yellow colour (overlapping by red and green colours) shown in Fig. 3(c) reveals the existence of microgel particles in the core. This can be attributed to the further segregation of PS chains and microgel particles in the initial microspheres upon the addition of water. In contrast, Fig. 3(e) shows that half microspheres or hollow shells were formed by using o-xylene as organic solvent after the addition of water. The half microspheres are further verified by the SEM image (Fig. 3f), which supports our above discussion that PS chains are quickly precipitated at the edge of the emulsion droplet, as a result of the partial engulfment of the emulsions droplet by the PS chains.
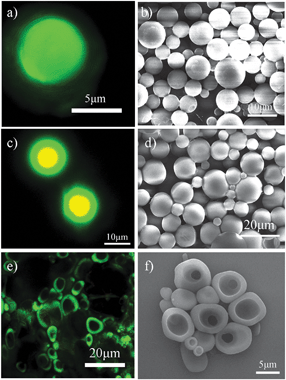 | ||
| Fig. 3 Confocal images of resultant microspheres based upon the use of different organic solvents after immersing in water for two days: (a) benzene, (c) toluene and (e) o-xylene. Lasers with wavelengths 543 and 408 nm were used; fluorescent microgel particles, red; oil with dissolved perylene, green. SEM images of produced microspheres based upon the use of different organic solvents after immersing in water for two days: (b) benzene, (d) toluene and (f) o-xylene. | ||
We also performed the controlled precipitation process using a biodegradable polymer, polycaprolactone (PCL, Mw ∼ 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, Aldrich), and got similar results. Biodegradable microspheres were obtained when both benzene and toluene were used as the oils. Meanwhile, using o-xylene as solvent only led to porous materials (Fig. S4–S6).
000 g mol−1, Aldrich), and got similar results. Biodegradable microspheres were obtained when both benzene and toluene were used as the oils. Meanwhile, using o-xylene as solvent only led to porous materials (Fig. S4–S6).
In summary, we have demonstrated the flexible formation of polymer microspheres by templating microgel-stabilized highly concentrated emulsions containing water insoluble polymer dissolved in organic solvents. After the solvent evaporation, microgel particles present in the interfacial area and the external aqueous phase initially form a solid support that dissolves rapidly upon addition of water to disperse the microspheres. This technique allows the production of large volumes of concentrated polymer microspheres (0.2 g in 5 mL solution) in a single step process. The microspheres size and morphology can be easily tuned by using organic solvents with different volatility. We believe that this technique is well-suited to producing particulate polymer particles for special applications.
The author T. Ngai would like to express special thanks to Professor Chi Wu, Dr Helmut Auweter and Professor Sven-Holger Behrens who led him to the field of colloids and materials. This work was financially supported by Hong Kong Special Administration Region (HKSAR) Earmarked Project (CUHK402707, 2160324) and the Direct Grant for Research 2008/09 of the Chinese University of Hong Kong (CUHK 2060371).
Notes and references
- S. Stolnik, L. Illum and S. Davis, Adv. Drug Delivery Rev., 1995, 16, 195 CrossRef CAS.
- A. Bell, Science, 2003, 299, 1688 CrossRef CAS.
- J. Wang, M. Gudiksen, X. Duan, Y. Cui and C. Lieber, Science, 2001, 293, 1455 CrossRef CAS.
- T. Piok, S. Gamerith, C. Gadermaier, H. Plank, F. Wenzl, S. Patil, R. Montenegro, T. Kietzke, D. Neher, U. Scherf, K. Landfester and E. List, Adv. Mater., 2003, 15, 800 CrossRef CAS.
- Y. Xia, B. Gates, Y. Yin and Y. Lu, Adv. Mater., 2000, 12, 693 CrossRef CAS.
- P. O'Donnell and J. McGinity, Adv. Drug Delivery Rev., 1997, 28, 25 CrossRef CAS.
- A. Henglein, Langmuir, 2001, 17, 2329 CrossRef CAS.
- C. Solans and H. Kunieda, Industrial Applications of Microemulsions, Marcel Dekker, Inc., 1997 Search PubMed.
- J. Schork, Y. Luo, W. Smulders, P. Russum, A. Butte and K. Fontenot, Adv. Polym. Sci., 2005, 175, 129 CAS.
- M. Antonietti and K. Tauer, Macromol. Chem. Phys., 2003, 204, 207 CrossRef CAS.
- S. Kawaguchi and K. Ito, Adv. Polym. Sci., 2005, 175, 299 CAS.
- M. Juba, ACS Symp. Ser., 1979, 104, 267 CAS.
- H. Bieringer, K. Flatau and D. Reese, Angew. Makromol. Chem., 1984, 123, 307 CrossRef.
- C. Boghina, C. Cincu, N. Marinescu, M. Marinescu, M. Dimonie, M. Lecca, G. Popescu, C. Porescu, A. Rosanu and M. Lungu, J. Macromol. Sci., Part A: Pure Appl. Chem., 1985, 22, 591 Search PubMed.
- H. Yow and A. Routh, Soft Matter, 2006, 2, 940 RSC.
- (a) A. Loxley and B. Vincent, J. Colloid Interface Sci., 1998, 208, 49 CrossRef CAS; (b) P. Dowding, R. Atkin, B. Vincent and P. Bouillot, Langmuir, 2004, 20, 11374 CrossRef CAS.
- (a) O. Cayre and S. Biggs, J. Mater. Chem., 2009, 19, 2724 RSC; (b) Z. Ao, Z. Yang, J. Wang, G. Zhang and T. Ngai, Langmuir, 2004, 20, 11374 CrossRef CAS.
- X. Gong, W. Wen and P. Shen, Langmuir, 2009, 25, 7072 CrossRef CAS.
- D. Horn and J. Rieger, Angew. Chem., Int. Ed., 2001, 40, 4330 CrossRef CAS.
- G. Zhang, A. Niu, S. Peng, M. Jiang, Y. Tu, M. Li and C. Wu, Acc. Chem. Res., 2001, 34, 249 CrossRef CAS.
- B. Binks, in Modern Aspects of Emulsion Science, ed. B. Binks, RSC, Cambridge, 1998 Search PubMed.
- A. Menner, R. Powell and A. Bismarck, Macromolecules, 2006, 39, 2034 CrossRef CAS.
- E. Ruckenstein, Adv. Polym. Sci., 1997, 127, 1 CAS.
- E. Ruckenstein and H. Li, Polym. Compos., 1997, 18, 320 Search PubMed.
- N. Cameron and D. Sherrington, Adv. Polym. Sci., 1996, 126, 163 CAS.
- Z. Li, T. Ming, J. Wang and T. Ngai, Angew. Chem., Int. Ed., 2009, 48, 8490 CrossRef CAS.
- Z. Li and T. Ngai, Langmuir, 2010, 26, 5088 CrossRef CAS.
- J. Texter, J. Dispersion Sci. Technol., 2001, 22, 499 CrossRef CAS.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Experimental details concerning polystyrene chains, microgel particles synthesis and characterization as well as high internal phase emulsion preparation and characterization. See DOI: 10.1039/c0cc02106g |
| This journal is © The Royal Society of Chemistry 2011 |
