On the performance of Cu-BTC metal organic framework for carbon tetrachloride gas removal†‡
Sofía
Calero
*,
Ana
Martín-Calvo
,
Said
Hamad
and
Elena
García-Pérez
Department of Physical, Chemical, and Natural Systems, University Pablo de Olavide, Seville, Spain. E-mail: scalero@upo.es; Fax: +34 95434 9814; Tel: +34 95497 7594
First published on 25th October 2010
Abstract
The performance of Cu-BTC metal organic framework for carbon tetrachloride removal from air has been studied using molecular simulations. According to our results, this material shows extremely high adsorption selectivity in favour of carbon tetrachloride. We demonstrate that this selectivity can be further enhanced by selective blockage of the framework.
Carbon tetrachloride had extensive industrial applications in the early 20th century, but it was phased out in the 2nd Montreal Protocol's revision in 1992 due to its adverse health effects. However, still today we find significant concentrations of carbon tetrachloride in air since it is involved in current industrial processes either for the lack of replacements or as undesirable by-products of other reactions. For this reason it is interesting to explore the possibilities to reduce the amount of carbon tetrachloride being released into the environment. Several recent reviews have reported the use of metal organic frameworks (MOFs) for treatment and remediation of pollutants and greenhouse gases in the environment.1–3 MOFs are a new class of highly porous crystalline materials formed by metal-oxide units that are joined by organic linkers through strong covalent bonds.4 Selective adsorption based mainly on the molecular sieving effect has been confirmed in several MOFs. Among them, with large surface area and high thermal stability, copper benzene tricarboxylate (Cu-BTC) has great potential as an adsorbent in industrial applications. In particular, this MOF has been suggested as useful for carbon dioxide capture and separation,5,6 removal of tetrahydrothiophene odorant from natural gas,3 or for the separation of polar components from nonpolar gases.7
Although application of Cu-BTC to gas separation and to high-density gas storage is being widely studied,2,3 no work has been undertaken to analyze its capacity for carbon tetrachloride separation from air. Here we report a series of simulation studies that establish a benchmark for the adsorption capacity in Cu-BTC across carbon tetrachloride and main air-component gases. These studies will serve to evaluate the potential of Cu-BTC as a material to be used in end-of-pipe technologies, especially for those processes in which clean technologies cannot be directly applied. Furthermore, they will provide insight into the properties of Cu-BTC that could make it most suited for this type of processes.
Cu-BTC was first reported in 1999 and named HKUST-1.8 Its framework is composed of dimeric cupric tricarboxylate units with a short Cu–Cu internuclear separation. Each metal completes its pseudo-octahedral coordination sphere with an axial water ligand opposite to the Cu–Cu vector.9 After removing water from the framework, it becomes an open three-dimensional porous structure with main channels of a square cross-section of about 9 Å diameter and tetrahedral side pockets of about 5 Å, which are connected to the main channels by triangular windows of about 3.5 Å in diameter. In this study we analyze adsorption based on four preferential sites: Site I, in the largest channels close to the copper atoms; Site II, at the center of the octahedral cages; and Site III, at the windows that allow communication between the octahedral cages and the main channels.10 More recently, an additional adsorption site, Site I′, which is located at the center of the main channels was also identified.11
We used Grand Canonical Monte Carlo simulations to compute single and multicomponent adsorption for carbon tetrachloride, argon, nitrogen, and oxygen at 298 K.§ The obtained isotherms for single argon, nitrogen, and oxygen are in very good agreement with available experimental and simulation data6,11–19 as shown in Fig. 1. The special arrangement of channels in MOF Cu-BTC together with open metal ligand sites offers dual adsorption behavior for these three molecules. Analysis of the occupancies of the individual adsorption sites indicates that argon adsorbs preferentially in the same sites as nitrogen and oxygen, i.e. in the octahedral cages (Site II), in the big cages (Site I′), and to a lower extent in the windows of the octahedral cages (Site III). As previously observed for nitrogen and oxygen,11 Site I remains empty over the entire range of pressures that we have analyzed for argon.
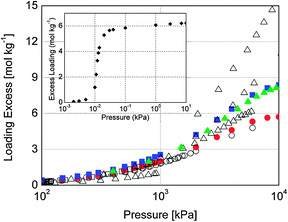 | ||
| Fig. 1 Comparison of simulated and previous adsorption isotherms of N2 (circles), O2 (squares) and argon (triangles) in Cu-BTC at 298 K. Our simulation results are represented by filled symbols and available previous data6,11–19 by open symbols. Absolute adsorption was converted to excess adsorption20 for comparison with experimental values. Inset figure provides the computed adsorption isotherm of CCl4 at 298 K. | ||
The computed adsorption isotherm for carbon tetrachloride shows a steep curve that might be attributed to clustering in the Cu-BTC pores21–23 (Fig. 1, inset). The obtained saturation value corresponds to 60 molecules of carbon tetrachloride per unit cell, all of them adsorbed in the main channels. To our knowledge, neither single nor multicomponent adsorption data involving carbon tetrachloride in Cu-BTC have been previously reported. The adsorption data provided in this work fill this gap and prioritize Cu-BTC over other materials in which carbon tetrachloride adsorption has already been reported24 (supporting information).
Some insight into the adsorption mechanism in Cu-BTC can be gleaned by observing the average occupation profiles for the adsorbed molecules. The profiles for carbon tetrachloride (Fig. 2) confirm that the preferential adsorption sites for this molecule are the big cages. At low pressures the molecule adsorbs preferentially in the center of the big cages (Site I′). However, at medium and high pressures the average occupation profiles reveal an additional site at the big cages, blocking the access to the windows that allow these cages to communicate with the octahedral pockets. Direct comparison of these profiles with the ones obtained for oxygen, nitrogen, and argon shows reverse adsorption trends for the latter. These three types of molecules firstly adsorb in the octahedral cages; once they are filled they adsorb in the windows, and finally they populate the big cages.
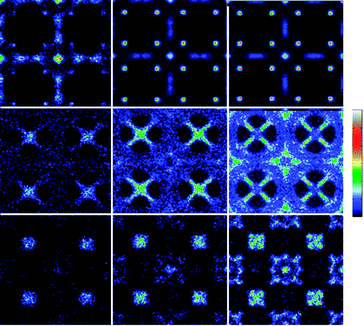 | ||
Fig. 2 Average occupation profiles in Cu-BTC obtained (from left to right) at low, medium and high pressures for single carbon tetrachloride adsorption (top), single oxygen adsorption (middle) and oxygen in the O2/N2/Ar/CCl4 mixture at a bulk partial fugacity ratio of 20.979![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 77.922 77.922![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.999 0.999![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1. The figure shows the average values of the projections of the center of mass coordinates over the x–y plane. Due to the cubic symmetry of Cu-BTC, the profile projected over the x–y plane is the same as the profiles obtained projecting the coordinates over the x–z and y–z planes. The relation between colour and occupation is shown in the bar situated on the right side of the figure. The same colour gradation (from dark blue to white) is employed in all figures, although the total number of molecules present in the unit cell is different for each calculation. 0.1. The figure shows the average values of the projections of the center of mass coordinates over the x–y plane. Due to the cubic symmetry of Cu-BTC, the profile projected over the x–y plane is the same as the profiles obtained projecting the coordinates over the x–z and y–z planes. The relation between colour and occupation is shown in the bar situated on the right side of the figure. The same colour gradation (from dark blue to white) is employed in all figures, although the total number of molecules present in the unit cell is different for each calculation. | ||
The adsorption isotherms in Cu-BTC for a four component mixture of oxygen, nitrogen, argon, and carbon tetrachloride were computed at 298 K. To mimic a sample of air containing carbon tetrachloride as contaminant the following composition was used for the mixture: 20.979% O2, 77.922% N2, 0.999% Ar, and 0.1% CCl4. While the average occupation profiles for carbon tetrachloride obtained from the mixture are very similar to those obtained for the single component, we found important differences for oxygen, nitrogen, and argon. Fig. 2 compares the average occupation profiles for oxygen as single component and in the multicomponent mixture. Nitrogen follows the same filling sequence as oxygen, and for the four component mixture, the argon adsorption in Cu-BTC is negligible. In the multicomponent mixture, the adsorption of oxygen, nitrogen, and argon is highly influenced by the presence of carbon tetrachloride adsorbing only in the sites that are not occupied by the latter. This, combined with the very low mole fraction of argon in the mixture leads to negligible adsorption of this component in Cu-BTC. As for oxygen and nitrogen, the main differences in adsorption are found at the windows that allow the octahedral cages to communicate with the main cages (now blocked by the carbon tetrachloride molecules), and also at the main cages (now filled with carbon tetrachloride molecules). Additional simulations showed that the adsorption selectivity of carbon tetrachloride over the main components of the air was even enhanced with ambient humidity (supporting information).
The self-diffusion coefficients for the different components of the quaternary mixture were computed by taking the slope of the mean-squared displacement at long times. Calculations were performed for the molecular loading obtained at 1000 kPa and 298 K i.e. 3 oxygen, 7 nitrogen, 0 argon, and 59 carbon tetrachloride molecules per unit cell. At 298 K the diffusion coefficient for the system with 3 oxygen molecules per unit cell (1.38 ± 0.02 10−8 m2 s−1) is lower than that obtained for the system with 7 nitrogen molecules per unit cell (1.91 ± 0.04 10−8 m2 s−1). This behaviour reverses in the quaternary mixture, though the diffusion coefficients for the two components decrease by three orders of magnitude. The computed self-diffusivity for oxygen and nitrogen in the quaternary mixture is 4.0 ± 0.1 10−11 m2 s−1 and 1.6 ± 0.1 10−11 m2 s−1, respectively. The slopes of the mean-squared displacements obtained for the 59 molecules of carbon tetrachloride at long times were almost zero, indicating very low self-diffusivity values for this molecule compared with the other two components.
Based on the differences in preferential adsorption sites for carbon tetrachloride and the air components, fine-tuning of the framework could be achieved for optimizing separation capacities. Hence, selective blockage of the octahedral pockets leads to extremely high adsorption selectivity in favor of carbon tetrachloride, as shown in Fig. 3.
![Adsorption selectivity of carbon tetrachloride over the different components of air at 298 K. The adsorption selectivity is defined as [xCCl4/yCCl4]/[xN/yN], where xi are the molar fractions in the adsorbed phase, yi the molar fractions in the bulk phase, and N indicates O2 (squares), N2 (circles) or Ar (triangles). The selectivity was obtained for the Cu-BTC structure with (empty symbols) and without (full symbols) selective blockage of the octahedral cages.](/image/article/2011/CC/c0cc02194f/c0cc02194f-f3.gif) | ||
| Fig. 3 Adsorption selectivity of carbon tetrachloride over the different components of air at 298 K. The adsorption selectivity is defined as [xCCl4/yCCl4]/[xN/yN], where xi are the molar fractions in the adsorbed phase, yi the molar fractions in the bulk phase, and N indicates O2 (squares), N2 (circles) or Ar (triangles). The selectivity was obtained for the Cu-BTC structure with (empty symbols) and without (full symbols) selective blockage of the octahedral cages. | ||
We conclude this work by highlighting that an effective adsorption material with long term viability in pollutants removal should combine (1) periodicity in the structure and (2) flexibility to achieve chemical functionalization and fine-tuning at a molecular level. Based on these properties, our results provide evidence of the Cu-BTC efficiency in carbon tetrachloride removal from air. We demonstrate that this efficacy can be further enhanced by selective blockage of the framework. Many technological processes require pollutants capture from air. Our results open an area of investigation in the field and indicate the potential of the Cu-BTC metal organic framework to complement and eventually replace other materials as dynamic adsorption media.
We acknowledge the funding viaCTQ2007-63229, CTQ2010-16077, and P07-FQM-02595 projects.
Notes and references
- D. Britt, D. Tranchemontagne and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 11623–11627 CrossRef CAS.
- S. Q. Ma and H. C. Zhou, Chem. Commun., 2010, 46, 44–53 RSC.
- U. Mueller, M. Schubert, F. Teich, H. Puetter, K. Schierle-Arndt and J. Pastre, J. Mater. Chem., 2006, 16, 626–636 RSC.
- H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Nature, 1999, 402, 276–279 CrossRef CAS.
- S. Cavenati, C. A. Grande and A. E. Rodrigues, Ind. Eng. Chem. Res., 2008, 47, 6333–6335 CrossRef CAS.
- A. Martin-Calvo, E. Garcia-Perez, J. M. Castillo and S. Calero, Phys. Chem. Chem. Phys., 2008, 10, 7085–7091 RSC.
- J. M. Castillo, T. J. H. Vlugt and S. Calero, J. Phys. Chem. C, 2008, 112, 15934–15939 CrossRef CAS.
- S. S. Y. Chui, S. M. F. Lo, J. P. H. Charmant, A. G. Orpen and I. D. Williams, Science, 1999, 283, 1148–1150 CrossRef CAS.
- C. Prestipino, L. Regli, J. G. Vitillo, F. Bonino, A. Damin, C. Lamberti, A. Zecchina, P. L. Solari, K. O. Kongshaug and S. Bordiga, Chem. Mater., 2006, 18, 1337–1346 CrossRef CAS.
- Y. Liu, C. M. Brown, D. A. Neumann, V. K. Peterson and C. J. Kepert, J. Alloys Compd., 2007, 446–447, 385–388 CrossRef CAS.
- E. Garcia-Perez, J. Gascon, V. Morales-Florez, J. M. Castillo, F. Kapteijn and S. Calero, Langmuir, 2009, 25, 1725–1731 CrossRef CAS.
- J. C. Liu, J. T. Culp, S. Natesakhawat, B. C. Bockrath, B. Zande, S. G. Sankar, G. Garberoglio and J. K. Johnson, J. Phys. Chem. C, 2007, 111, 9305–9313 CrossRef CAS.
- P. Chowdhury, C. Bikkina, D. Meister, F. Dreisbach and S. Gumma, Microporous Mesoporous Mater., 2009, 117, 406–413 CrossRef CAS.
- G. Garberoglio, A. I. Skoulidas and J. K. Johnson, J. Phys. Chem. B, 2005, 109, 13094–13103 CrossRef CAS.
- J. R. Karra and K. S. Walton, Langmuir, 2008, 24, 8620–8626 CrossRef CAS.
- A. I. Skoulidas, J. Am. Chem. Soc., 2004, 126, 1356–1357 CrossRef.
- A. I. Skoulidas and D. S. Sholl, J. Phys. Chem. B, 2005, 109, 15760–15768 CrossRef CAS.
- Q. M. Wang, D. M. Shen, M. Bulow, M. L. Lau, S. G. Deng, F. R. Fitch, N. O. Lemcoff and J. Semanscin, Microporous Mesoporous Mater., 2002, 55, 217–230 CrossRef CAS.
- Q. Y. Yang, C. Y. Xue, C. L. Zhong and J. F. Chen, AIChE J., 2007, 53, 2832–2840 CrossRef CAS.
- T. Duren, L. Sarkisov, O. M. Yaghi and R. Q. Snurr, Langmuir, 2004, 20, 2683–2689 CrossRef CAS.
- K. S. Walton, A. R. Millward, D. Dubbeldam, H. Frost, J. J. Low, O. M. Yaghi and R. Q. Snurr, J. Am. Chem. Soc., 2008, 130, 406 CrossRef CAS.
- R. Krishna and J. A. van Baten, Langmuir, 2010, 26, 3981–3992 CrossRef CAS.
- R. Krishna and J. M. van Baten, Langmuir, 2010, 26, 8450–8463 CrossRef CAS.
- X. S. Zhao, Q. Ma and G. Q. M. Lu, Energy Fuels, 1998, 12, 1051–1054 CrossRef CAS.
- S. L. Mayo, B. D. Olafson and W. A. Goddard, J. Phys. Chem., 1990, 94, 8897–8909 CrossRef CAS.
- A. K. Rappe, C. J. Casewit, K. S. Colwell, W. A. Goddard and W. M. Skiff, J. Am. Chem. Soc., 1992, 114, 10024–10035 CrossRef CAS.
- D. Farrusseng, C. Daniel, C. Gaudillere, U. Ravon, Y. Schuurman, C. Mirodatos, D. Dubbeldam, H. Frost and R. Q. Snurr, Langmuir, 2009, 25, 7383–7388 CrossRef CAS.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Table 1 listing all charges and intermolecular parameters used in this work, Fig. 1S showing comparison with other materials and Fig. 2S comparing adsorption selectivities with and without ambient humidity. See DOI: 10.1039/c0cc02194f |
| § Simulation Methods and Models: The adsorption isotherms were obtained from Grand Canonical Monte Carlo simulations. The Henry coefficients and energies of adsorption were computed using Monte Carlo in the NVT ensemble at 298 K. Cu-BTC was modeled as a rigid structure with Lennard-Jones parameters taken from ref. 25 except those for Cu that were taken from ref. 26. Atomic charges were taken from ref. 27 and Lorentz-Berthelot mixing rules were used to calculate mixed Lennard-Jones parameters. One unit cell of Cu-BTC (a = b = c = 26.343 Å) was used in our simulations. We obtained a helium void fraction of 0.76. The interactions between guest molecules with the Cu-BTC host framework are modeled by Lennard-Jones and Coulombic potentials. Detailed information about these methods can be found elsewhere.11 Adsorbates were considered as rigid molecules. For Ar, N2, and O2 we used models and potentials that have been successfully employed to describe the adsorption in Cu-BTC.6, 11 The Lennard-Jones parameters for Ar, O2, N2, and CCl4 were fitted to reproduce the equilibrium vapor–liquid equilibrium curve. Table 1 in the supporting information collects the charges and intermolecular parameters used in this work. |
| This journal is © The Royal Society of Chemistry 2011 |
