A ratiometric probe for the selective time-gated luminescence detection of potassium in water†‡
Evan A.
Weitz
and
Valérie C.
Pierre
*
Department of Chemistry, University of Minnesota, 207 Pleasant Street SE, Minneapolis, MN 55455, USA. E-mail: pierre@umn.edu; Fax: (+1) 612 626 7541; Tel: (+1) 612 625 0921
First published on 19th November 2010
Abstract
A europium probe for the ratiometric detection of potassium in water is presented. This probe demonstrates high sensitivity, with an affinity for K+ in the mM range, and high selectivity for K+ over Na+, Ca2+, Mg2+ and Li+. The long luminescence lifetime of the probe and its large Stokes shift further enable accurate determination of the concentration of K+ in complex aqueous media.
The development of luminescent probes for the imaging of potassium inside and outside living cells remains an active and challenging area of research. Given the importance of the alkali ions in a multitude of biological processes, including regulation of membrane polarization and osmotic pressure, there is a need for practical sensitive and selective luminescent probes for K+. However, sensitivity and selectivity alone are not sufficient to ensure that a probe be valuable for neuroimaging. The probe must also turn on and off with speed comparable to the flux of the alkali ions across the cell membrane. This requires that the kinetics of binding and dissociation of the K+-probe adduct also be comparable to the rate of flow of the ions during the propagation of an action potential. Such probes, presenting high sensitivity and selectivity with fast binding and dissociation kinetics, have yet to be developed. Herein, we present a step towards this goal with the development of a ratiometricprobe for K+ that displays high selectivity and sensitivity in the mM range by incorporating a phenanthridine-based lariat ether.
The majority of luminescent probes for potassium developed thus far rely on PeT (photo electron transfer) quenching of a chromophore or an antenna by a nearby amine-containing receptor.1–4 The selectivity of these probes are thus entirely based on that of the receptor. They can be divided into two classes: crown- or lariat-ethers, which demonstrate limited selectivity for K+ over Na+ but rapid turn-on kinetics; and cryptands which show improved selectivity but also significantly slower binding and dissociation rates.5 This slow cation release in turn prevents the application of the cryptand-based probes for the imaging of very rapid fluctuation in K+ concentrations, such as those that occur during an action potential.
We aimed to improve the selectivity of lariat-ether based receptors without affecting their kinetics of binding so as to develop probes that could potentially be used to image the action potentials and other processes which involve rapid fluctuation in K+ levels. In this regard, our group recently developed a highly selective luminescent probe based on the cation–π interaction and the dependence of the terbium luminescence on the distance separating the metal from its antenna.6 Unfortunately, the effectiveness of this probe in aqueous solutions was limited. Based on the work of the Parker group, we reasoned that a selective turn-on probe could be developed via direct coordination of the potassium ion by a phenanthridine antenna. Parker and coworkers previously reported that the excitation profile of a macrocyclic EuIII complex bearing a phenanthridine antenna varied significantly with the latter's protonation state.7 Protonation of the phenanthridine resulted in a complex that could be excited at significantly longer wavelength. Moreover, the presence on an isosbestic point in the excitation profiles suggested that a phenanthridine antenna would lead to a ratiometric probe. A ratiometric probe would be significantly more valuable in the accurate measurement of potassium concentration in living cells since the concentration of such a probe in biological studies can be readily determined.
Our probe, Eu-KPhen (1) (Fig. 1) thus incorporates a macrocyclic Eu complex for improved stability in biological media, a diaza-18-crown-6 receptor, and a phenanthridine antenna conjugated to the crown ether at the 4 position. Conjugation at this position enables the phenanthridine to coordinate the potassium encapsulated within the crown ether. We hypothesized that this complexation would modify the excited energy levels of the antenna, thus affecting the excitation profile of the Eu complex resulting in a ratiometric turn-on probe (Fig. 1).
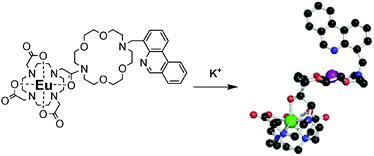 | ||
| Fig. 1 Chemical structure and mode of action of the K+ probe Eu-KPhen (1). | ||
The probe Eu-KPhen (1) was synthesized in thirteen steps according to Scheme 1 (see ESI for details).‡ The europium-centered time-gated luminescence intensity of Eu-KPhen (1) increases up to two fold upon titration of potassium ion in water at neutral pH when excited at 267 nm (Fig. 2). Remarkably, the binding affinity of Eu-KPhen (1) for K+ in water is 26 ± 0.8 μM−1. This is notably higher than the affinity of the corresponding diaza-18-crown-6 and lariat ethers for the alkali cation which are typically in the mM−1 range,8 and therefore suggests that at least partial coordination of the potassium by the phenanthridine's nitrogen is occurring. Advantageously, this higher binding affinity enables the detection of K+ in concentrations ranging from 0–10 mM in water at physiological pH. As such, the probe is ideally suited for imaging the alkali cation in the extracellular environment, where its concentration ranges typically ranges from 3.5–5.3 mM.9 It should be noted that for application in neuroimaging, it is more beneficial to image the extracellular K+ level than intracellular ones, as the bigger concentration change during an action potential will occur extracellularly. In this respect, Eu-KPhen (1) is a significant improvement over the previously published lanthanide-based probes of Gunnlaugsson3 and Wong4 which enable detection of aqueous K+ in the 0.1–1 M range but which displayed negligible luminescence enhancement at the mM ranges of extracellular significance.
 | ||
| Scheme 1 Synthesis of Eu-KPhen (1). Reagents and conditions: (a) PhB(OH)2, (PPh3)4Pd, Na2CO3, toluene, H2O, reflux, 13 h, 92.9%; (b) 36 bar H2, Pd/C, CH3OH, 21 h, 99.0%; (c) HCOOH, N2, reflux, 52 h; (d) PPA, 150° C, 3 h, 68.5% over 2 steps; (e) NBS, hν, benzene, rt, 51.4%; (f) Cs2CO3, CH3CN, 50° C, 96 h, 44.8%; (g) TFA, CH2Cl2, rt, 1.5 h, 83.2%; (h) chloroacetylchloride, N2, CH2Cl2, 0° C, 4 h, 63.5%; (i) cyclen, Cs2CO3, CH3CN, Ar, rt, 88 h; (j) t-BuO2CCH2Br, CH3CN, rt, 59.5% over 2 steps; (k) TFA, CH2Cl2, rt, 1 h, 80.9%; (l) EuCl3, H2O, pH 7, reflux, 19 h, 99.9%. See ESI for details.‡ | ||
![Relative time-delayed luminescence of Eu-KPhen (1) as a function of K+ concentration (filled black squares) and Na+ (open red circles). Error bars represent standard deviation (s.d.), n = 3. Experimental conditions: [Eu-KPhen] = 50.0 μM, 25 mM aqueous OAc−buffer pH 7, excitation at 267 nm, emission at 593 nm, time delay = 0.1 ms, T = 20 °C.](/image/article/2011/CC/c0cc02637a/c0cc02637a-f2.gif) | ||
| Fig. 2 Relative time-delayed luminescence of Eu-KPhen (1) as a function of K+ concentration (filled black squares) and Na+ (open red circles). Error bars represent standard deviation (s.d.), n = 3. Experimental conditions: [Eu-KPhen] = 50.0 μM, 25 mM aqueous OAc−buffer pH 7, excitation at 267 nm, emission at 593 nm, time delay = 0.1 ms, T = 20 °C. | ||
Eu-KPhen (1) has three other significant advantages over previously reported luminescent probes for potassium: (1) it is ratiometric, (2) it has a long luminescence lifetime, and (3) it displays a very large pseudo-Stokes shift between the absorption of the antenna and the emission of the lanthanide. As can be seen in the excitation profile of the europium complex as a function of the concentration of added potassium (Fig. 3), if the europium complex is excited at shorter wavelengths such as 265 nm, then its emission depends on the concentration of K+. Excitation at 400 nm, however, yields a europium centered emission which is independent of the concentration of potassium. The two excitation wavelengths can thus be used ratiometrically to determine quantitatively and thus with significantly higher accuracy, the concentration of potassium in a biological medium. Excitation at 400 nm yields the concentration of the europium probe. From the binding affinity of Eu-KPhen for K+ (26 μM−1), the emission intensity of Eu upon excitation at 267 nm enables accurate determination of the concentration of the alkali cation.
![Metal luminescence excitation spectra of Eu-KPhen (1) upon titration of K+. Experimental conditions: [Eu-KPhen] = 50.0 μM, 25 mM aqueous OAc−buffer pH 7, emission 593 nm, time delay = 0.1 ms, T = 20 °C.](/image/article/2011/CC/c0cc02637a/c0cc02637a-f3.gif) | ||
| Fig. 3 Metal luminescence excitation spectra of Eu-KPhen (1) upon titration of K+. Experimental conditions: [Eu-KPhen] = 50.0 μM, 25 mM aqueous OAc−buffer pH 7, emission 593 nm, time delay = 0.1 ms, T = 20 °C. | ||
Comparison with the UV-visible absorption spectrum of the phenanthridine moiety (see ESI‡) indicates that the responsive wavelengths of excitation of the probe Eu-KPhen (250–350 nm) correspond to the absorbance of the phenanthridine moiety. Coordination of K+ by the phenanthridine thus likely affects the efficiency of energy transfer from the antenna to the lanthanide. Correspondingly, the intensity of Eu emission is a function of the K+ concentration, as observed in the titrations (Fig. 2).
Additionally, the long luminescence lifetime of europium (>1 ms) readily enables time-gated luminescence imaging, whereby any background luminescence from a biological media, which typically has a lifetime shorter than the μs range, is advantageously cut-off.10 This techniques further improves the accuracy of the probe in biological applications. Moreover, the large Stokes' shift of the complex (excitation at 267 nm, emission at 593 nm) and the negligible overlap between the absorption and the emission spectra, limit any self-absorption problems at high concentration. Taken together, these advantages render Eu-KPhen (1) a promising candidate for imaging extra-neuronal potassium fluxes during an action potential.
For application in neuroimaging, however, a potassium probe must also be highly selective for potassium over sodium. The extracellular concentration of potassium is 3.5–5.3 mM whereas that of sodium is 135–148 mM. There is therefore a 30–40 fold excess of sodium in the extracellular matrix. As can be seen in Fig. 3, the selectivity of Eu-KPhen (1) for K+ over Na+ is significantly higher than that of most crown and lariat ethers, which typically demonstrate 5–10 fold selectivity for the larger alkali cation.8 The time-gated luminescence intensity of the probes increases 83% upon addition of K+ and plateaus at 10 mM, whereas negligible turn on is observed with a similar range of sodium concentrations (Fig. 3). A typical extracellular concentration of 137 mM Na+, increases the luminescence by 35%. In comparison, the luminescence intensity of Tsien's PBFI probe11 and Crossley's CD18 probe1 in the presence of 10 mM K+ is similar to that of 125 mM Na+. The higher selectivity for K+vs.Na+ and the ratiometric aspect of Eu-KPhen (1) renders it a more promising candidate for the intended long-term application of imaging neuron activity.
The selectivity of Eu-KPhen (1) for K+ over other physiological cations including Ca2+, Mg2+ and Li+ is shown in Fig. 4. Typical serum concentration of the alkali earth9Ca2+ (2.47 mM) and Mg2+ (0.83 mM) do not significantly affect the response of the probe. Ca2+ increases the luminescence intensity by 9% whereas Mg2+ increases it by 3%. Addition of a large excess of Li+ (50.0 mM) also has negligible influence on the luminescence of the probe. Importantly, Importantly, the subsequent addition of 20 mM KOAc restores the two-fold increase in luminescence, demonstrating that the presence of competing cations does not affect the determination of K+ concentration. (see ESI‡).
![Selectivity of Eu-KPhen (1) for K+ over physiological cations at typical serum concentrations. Time-gated emission spectra (1) in water (black) and in the presence of 50.0 mM Li+ (blue), 0.83 mM Mg2+ (green), 2.47 mM Ca2+ (yellow), 107 mM Na+ (orange) and 10 mM K+ (red). Experimental conditions: [Eu-KPhen] = 50.0 μM, 25 mM aqueous OAc−buffer, pH 7, excitation at 267 nm, emission at 593 nm, time delay = 0.1 ms, T = 20 °C.](/image/article/2011/CC/c0cc02637a/c0cc02637a-f4.gif) | ||
| Fig. 4 Selectivity of Eu-KPhen (1) for K+ over physiological cations at typical serum concentrations. Time-gated emission spectra (1) in water (black) and in the presence of 50.0 mM Li+ (blue), 0.83 mM Mg2+ (green), 2.47 mM Ca2+ (yellow), 107 mM Na+ (orange) and 10 mM K+ (red). Experimental conditions: [Eu-KPhen] = 50.0 μM, 25 mM aqueous OAc−buffer, pH 7, excitation at 267 nm, emission at 593 nm, time delay = 0.1 ms, T = 20 °C. | ||
In conclusion, we have developed a novel lanthanide-based luminescent probe for the detection of potassium in water at neutral pH. This probe presents high affinity for K+ in the mM range and good selectivity over Na+ and physiologically relevant cations including Ca2+, Mg2+ and Li+. Advantageously, this probe presents long luminescence lifetime which readily enables time-gating imaging. Notably, it is also the first ratiometric luminescent probe for K+; the Eu-centered luminescence is dependent on the K+ concentration if the probe is excited at 265 nm but independent of it if excited at 400 nm. The ratiometric response enables a more accurate determination of K+ levels in solutions.
This research was supported by the University of Minnesota.
Notes and references
- R. Crossley, Z. Goolamali, J. J. Gosper and P. G. Sammes, J. Chem. Soc., Perkin Trans. 2, 1994, 513–520 RSC
.
- R. Crossley, Z. Goolamali and P. G. Sammes, J. Chem. Soc., Perkin Trans. 2, 1994, 1615–1623 RSC
; A. P. de Silva, H. Q. N. Gunaratne and K. R. A. S. Sandanayake, Tetrahedron Lett., 1990, 31, 5193–5196 CrossRef
; A. P. deSilva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515–1566 CrossRef
.
- T. Gunnlaugsson and J. P. Leonard, Chem. Commun., 2003, 2424–2425 RSC
; T. Gunnlaugsson and J. P. Leonard, J. Chem. Soc., Dalton Trans., 2005, 3204–3212 RSC
.
- C. Li, G. L. Law and W. T. Wong, Org. Lett., 2004, 6, 4841–4844 CrossRef CAS
.
- P. Padmawar, X. M. Yao, O. Bloch, G. T. Manley and A. S. Verkman, Nat. Methods, 2005, 2, 825–827 CrossRef CAS
; W. Namkung, P. Padmawar, A. D. Mills and A. S. Verkman, J. Am. Chem. Soc., 2008, 130, 7794–7795 CrossRef CAS
; M. Magzoub, P. Padmawar, J. A. Dix and A. S. Verkman, J. Phys. Chem. B, 2006, 110, 21216–21221 CrossRef CAS
; R. C. Helgeson, B. P. Czech, E. Chapoteau, C. R. Gebauer, A. Kumar and D. J. Cram, J. Am. Chem. Soc., 1989, 111, 6339–6350 CrossRef CAS
; H. R. He, M. A. Mortellaro, M. J. P. Leiner, R. J. Fraatz and J. K. Tusa, J. Am. Chem. Soc., 2003, 125, 1468–1469 CrossRef CAS
.
- A. Thibon and V. C. Pierre, J. Am. Chem. Soc., 2009, 131, 434–435 CrossRef CAS
.
- D. Parker, K. Senanayake and J. A. G. Williams, Chem. Commun., 1997, 1777–1778 RSC
; D. Parker, P. K. Senanayake and J. A. G. Williams, J. Chem. Soc., Perkin Trans. 2, 1998, 2129–2139 RSC
; D. Parker and J. A. G. Williams, Chem. Commun., 1998, 245–246 RSC
.
- R. M. Izatt, K. Pawlak, J. S. Bradshaw and R. L. Bruening, Chem. Rev., 1991, 91, 1721–2085 CrossRef CAS
; V. J. Gatto and G. W. Gokel, J. Am. Chem. Soc., 1984, 106, 8240–8244 CrossRef CAS
; V. J. Gatto, K. A. Arnold, A. M. Viscariello, S. R. Miller and G. W. Gokel, Tetrahedron Lett., 1986, 27, 327–330 CrossRef CAS
; V. J. Gatto, K. A. Arnold, A. M. Viscariello, S. R. Miller, C. R. Morgan and G. W. Gokel, J. Org. Chem., 1986, 51, 5373–5384 CrossRef CAS
.
-
J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer, New York, 2006 Search PubMed
.
- A. Thibon and V. C. Pierre, Anal. Bioanal. Chem., 2009, 394, 107–120 CrossRef CAS
; E. J. New, D. Parker, D. G. Smith and J. W. Walton, Curr. Opin. Chem. Biol., 2010, 14, 238–246 CrossRef CAS
; J. C. G. Bunzli, Chem. Rev., 2010, 110, 2729–2755 CrossRef
.
- A. Minta and R. Y. Tsien, J. Biol. Chem., 1989, 264, 19449–19457
.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Detailed experimental procedure for the synthesis of Eu-KPhen and titrations. See DOI: 10.1039/c0cc02637a |
| This journal is © The Royal Society of Chemistry 2011 |
