Biomimetic mineralization of vertical N-doped carbon nanotubes†‡
Won Jun
Lee
,
Duck Hyun
Lee
,
Tae Hee
Han
,
Sun Hwa
Lee
,
Hyoung-Seok
Moon
,
Jin Ah
Lee
and
Sang Ouk
Kim
*
Department of Materials Science and Engineering, KAIST, Daejeon, 305-701, Republic of Korea. E-mail: sangouk.kim@kaist.ac.kr; Fax: +82 42 350 3310; Tel: +82 42 350 3339
First published on 16th November 2010
Abstract
Biomimetic mineralization of vertical N-doped carbon nanotubes is demonstrated as a straightforward route for carbon-based mineral nanocomposites. The N-doped sites along the carbon nanotube backbone play the role of nucleation sites for mineralization.
Biomineralization is a natural self-assembly that generates complex mineral organization at organic template surfaces.1 To date, more than 60 species of biominerals have been reported and produced with desired complex architectures.2 In general, biomineralization occurs in an environmentally benign process in neutral pH aqueous media at ambient pressure and temperature.3 Furthermore, unlike conventional vapor phase deposition, biomineralization enables low cost, rapid mineral deposition with low energy consumption. Nevertheless, biomineralization has only partially succeeded in creating high performance composites thus far. The poor strength and low thermal or chemical stability of organic templates, such as peptides or synthetic nitrogen containing polymers, deteriorate the ultimate strength and sustainability of biomineralized structures.4
Carbon Nanotubes (CNTs) are allotropes of graphitic sp2 hybridized carbon atoms wrapped into a cylindrical morphology. Over the past 15 years since their discovery, CNTs have received tremendous research interest owing to their excellent material properties, including high mechanical properties, superior thermal or electrical conductivity, and large surface area.5 However, the low surface energy and the chemical inertness of CNTs have limited the ultimate utilization of their extraordinary properties. To address these shortcomings, various chemical functionalization methods have been developed.6 However, such functionalization may damage the graphitic conjugated structure of CNTs, significantly degrading the material properties.7
Here we introduce a new biomimetic mineralization route employing N-doped carbon nanotubes (NCNTs) as highly stable template materials. NCNTs are a new type of carbon nanotubes (CNTs), where N atoms partially substitute a part of carbon atoms in the graphitic side wall of CNTs. The electron rich nitrogen may enhance the electrical conductivity as well as the surface energy of CNTs.8 In this work, the substitutionally doped quaternary and pyridinic N atoms in the graphitic plane of NCNTs are exploited as the nucleation site for biomimetic mineralization. Taking advantage of the nitrogen doped sites along the CNT backbone, silica (SiO2) and calcium carbonate (CaCO3) nanoshells can be readily deposited on a vertical NCNT array (Fig. 1a). Owing to the superior material properties of NCNTs and straightforward processing via biomimetic mineralization, our approach offers a new opportunity for carbon-based mineral nanocomposites.
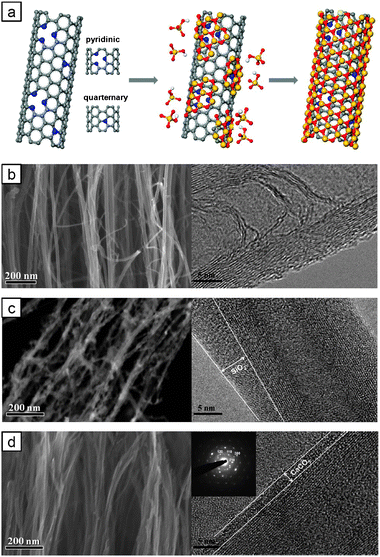 | ||
| Fig. 1 (a) Biomimetic mineralization of NCNTs. N-Doping sites act as nucleation sites. SEM (left) and TEM (right) images of (b) NCNTs, (c) SiO2 coated NCNTs and (d) CaCO3 coated NCNTs. | ||
Vertical NCNTs were grown by plasma-enhanced chemical vapor deposition (PECVD) as described elsewhere.9,10Fig. 1b presents the field emission scanning electron microscopy (FE-SEM) and high-resolution transmission electron microscopy (HR-TEM) images of the synthesized NCNTs. Fig. 1b shows the typical bamboo-like structure of N-doped, multi-walled CNTs. The presence of nitrogen in the NCNTs was confirmed by energy-dispersive spectroscopy (EDX) (see ESI‡, Fig. S1a). Our typical growth condition generated the NCNTs with a length of 10–20 μm and a diameter of 15–20 nm.
Fig. 1c shows the FE-SEM and HR-TEM images of the SiO2 coated NCNTs prepared by our biomimetic mineralization. The HR-TEM image shows that the NCNTs were coated by a SiO2 nanoshell with a thickness of 4 ± 1 nm. The presence of Si element could be confirmed by EDX (see ESI‡, Table S1). Previous SiO2 coating of CNTs has been performed by surfactant mediated coating11 or electrodeposition under external fields,12 or sol–gel technique with a sintering process.13 Those approaches have been employed for undoped pristine CNTs or oxygenated CNTs. To verify the unique role of N-doping in our biomimetic mineralization, undoped or acid-treated oxygenated CNTs were treated by our biomimetic mineralization process. Undoped CNTs were hardly wet by the aqueous mineralization solutions and the resultant CNTs were sparsely covered by SiO2 particles (see ESI‡, Fig. S2 and S3 and Table S1). Hydrophilic oxygenated CNTs prepared by acid treatment could not be mineralized, despite moderate hydrophilicity (see ESI‡, Fig. S2 and S3 and Table S1). These results confirm that N-doping plays a critical role in our biomimetic process.
Fig. 1d shows the FE-SEM, HR-TEM and Selected Area Electron Diffraction (SAED) pattern (inset) images of the CaCO3 coated NCNTs.14EDX measurement detected a Ca element (see ESI‡, Fig. S1c). The HR-TEM image shows that the NCNTs were coated by calcite nanoshell with a thickness of 2 ± 1 nm. The SAED pattern shows the typical [110] crystal zone of calcite. We also confirmed that undoped or oxygenated CNTs could not be CaCO3 mineralized via our biomimetic process (see ESI‡, Fig. S2 and S3 and Table S1).
Our facile biomimetic mineralization of NCNTs was accomplished by a simple dip-coating process (Fig. 2a).
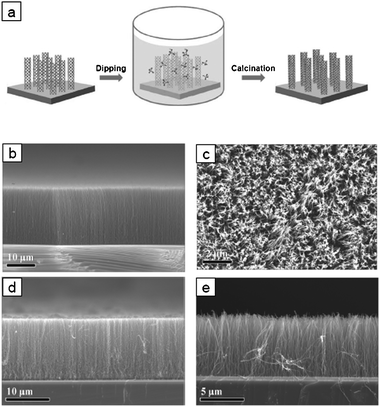 | ||
| Fig. 2 (a) Biomimetic mineralization of vertical NCNTs. (b) Cross-sectional and (c) plane-view SEM images of vertical NCNTs. SEM images of (d) SiO2 and (e) CaCO3 coated vertical NCNTs. | ||
Firstly, vertical NCNTs were dipped in ethanol for 1 min, and transferred to an aqueous mineral solution (typically a 6 mL solution of Na2SiO3·nH2O or CaCO3·H2O bubbled with CO2) including a coupling agent (Tris·HCl and NH3) at a pH of 5.5–8.5. The experimental details are described in ESI.‡ The prior ethanol dipping promoted the wetting of the NCNTs with the aqueous mineralization solution. After biomimetic mineralization, evaporation of the aqueous solution may cause capillary forces to act among the NCNT strands and, thus, create a collapsed morphology of NCNT forests.15 Such a collapse can be avoided by a rapid evaporation of the residual solution under vacuum. Fig. 2b and c show the cross-sectional and plane view SEM images of the vertical NCNTs before mineralization. Highly aligned, ∼20 mm tall, vertical NCNTs were grown on the silicon substrate. The plane view image of the top-surface confirms that the NCNTs grew densely but individually separated. Fig. 2d and e present the SiO2 and CaCO3 coated vertical NCNTs prepared by biomimetic mineralization. Owing to the nanoscale thickness of the mineralized shells, the vertical mineralized NCNT strands were individually separated (see ESI‡, Fig. S4). The penetration speed of mineralization solution could be controlled by masking the vertical NCNTs. The mask retarded penetration of mineralization solution, thereby producing half-coated silica composites (see ESI‡, Fig. S5a). By controlling the penetration speed, we have assured that vertical NCNTs can be uniformly mineralized along their entire lengths.
Fig. 3 compares the X-ray photoelectron spectroscopy (XPS) spectra of the bare NCNTs and biomimetic mineralized NCNTs. Fig. 3a shows that the bare NCNT spectrum exhibited a strong nitrogen incorporated carbon peak (CN, 285.5 eV). After mineralization, the CN peak disappeared and the C1s peak decreased (Fig. 3a). These XPS results suggest the specific interaction between the N-doped sites of the NCNTs and the mineral shell. Fig. 3b shows the nitrogen XPS spectra. The deconvoluted spectra of the bare NCNTs showed pyridinic N (NP, 398 eV), quaternary N (NQ, 400.8 eV), and nitrogen oxide (NOX, 402–406 eV) peaks.
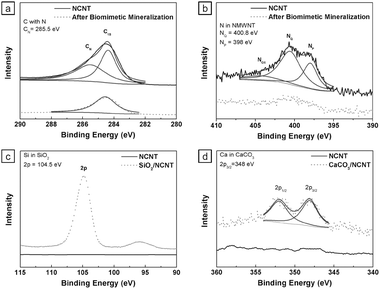 | ||
| Fig. 3 XPS spectra of NCNTs before and after biomimetic mineralization. (a) C peaks for C with N (CN) and C1s. (b) N peaks for pyridinic N (NP), quaternary N (NQ), and N-oxides (NOX). (c) Si peak for Si2P and (d) Ca peaks for Ca2P. | ||
In contrast, the mineralized NCNTs demonstrated a suppression of all nitrogen peaks. These results strongly suggest that the NP and NQ doping sites in the NCNTs provide the major nucleation sites for biomimetic mineralization (see ESI‡, Fig. S3). While the SiO2 coated NCNTs demonstrated a strong Si2P peak at 104 eV (Fig. 3c), the CaCO3 coated NCNTs demonstrated two bimodal calcium peaks at 347–348 eV (Fig. 3d).
The X-ray diffraction (XRD) patterns of the bare NCNTs, SiO2 coated NCNTs, and CaCO3 coated NCNTs are compared in Fig. 4. The diffraction peaks for calcite lattice are prominent, as indicated by the characteristic peaks at 29.4°, 35.9°, 39.4°, 43.1°, 47.5°, and 48.5°, which correspond to the calcite lattice planes of (104), (110), (113), (202), (018), and (116), respectively (Ref JCPDS Card No. 00-005-0586). Since SiO2 is amorphous, no noticeable diffraction peaks were observed. The broad peak at 2θ = 43° is attributed to the (103) plane of CNT graphitic structure.
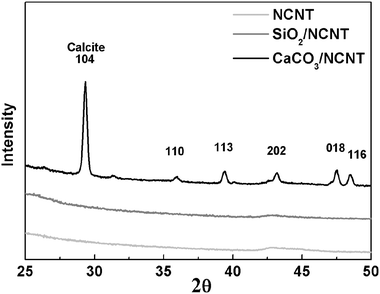 | ||
| Fig. 4 XRD patterns for bare NCNTs, SiO2 coated NCNTs, and CaCO3 coated NCNTs. | ||
In conclusion, we have demonstrated the biomimetic mineralization of NCNTs. The NCNT template offers a new perspective for mineralization and carbon based nanocomposites under mild processing conditions. The detailed structural analysis revealed that the N-doping sites of the NCNTs play the role of nucleation sites for biomimetic mineralization. Our approach not only provides a novel route for nanostructured materials but also suggests that the graphitic N-doping sites can play a significant role in biomimetic mineralization.
This work was supported by the National Research Laboratory Program (R0A-2008-000-20057-0), and World Premier Materials (WPM) program funded by the Korean government.
Notes and references
- (a) S. Mann, Nature, 1988, 332, 119 CrossRef CAS; (b) A. H. Heuer, D. J. Fink, V. J. Laraia, J. A. Arias, P. D. Calvert, K. Kendall, G. L. Messing, J. Blackwell, P. C. Rieke, D. H. Thompson, A. P. Wheeler, A. Veis and A. I. Caplan, Science, 1992, 255, 1098 CAS; (c) I. W. Hamley, Angew. Chem., Int. Ed., 2003, 42, 1692 CrossRef CAS; (d) S. Mann, Chem. Commun., 2004, 1 RSC; (e) C. Sanchez, H. Arribart and M. M. G. Guille, Nat. Mater., 2005, 4, 277 CrossRef CAS; (f) A.-W. Xu, Y. Ma and H. Cölfen, J. Mater. Chem., 2007, 17, 415 RSC; (g) J. Aizenberg, MRS Bull., 2010, 35, 323 CAS.
- (a) G. Xu, N. Yao, I. A. Aksay and J. T. Groves, J. Am. Chem. Soc., 1998, 120, 11977 CrossRef CAS; (b) M. Sumper and E. Brunner, Adv. Funct. Mater., 2006, 16, 17 CrossRef CAS; (c) Y. Fan, K. Duan and R. Wang, Biomaterials, 2005, 26, 1623 CrossRef CAS; (d) Y. Oaki and H. Imai, Chem. Commun., 2005, 6011 RSC; (e) S. Mann, N. H. C. Sparks, R. B. Frankel, D. A. Bazylinski and H. W. Jannasch, Nature, 1990, 343, 258 CrossRef CAS; (f) S.-H. Yu, H. Cölfen, K. Tauer and M. Antonietti, Nat. Mater., 2005, 4, 51 CrossRef CAS; (g) H. C. Lichtenegger, T. Schöberl, M. H. Bartl, H. Waite and G. D. Stucky, Science, 2002, 298, 389 CrossRef CAS.
- (a) S. Weiner and L. Addadi, Science, 2002, 298, 375 CrossRef CAS; (b) H. Cölfen and S. Mann, Angew. Chem., Int. Ed., 2003, 42, 2350 CrossRef; (c) N. Kröger and K. H. Sandhage, MRS Bull., 2010, 35, 122 CAS.
- (a) R. Langer, Science, 1990, 249, 1527 CrossRef CAS; (b) J. R. Johnson III, J. Spikowski and D. A. Schiraldi, ACS Appl. Mater. Interfaces, 2009, 1, 1305 CrossRef; (c) J. Rodríguez-Hernández, J. Babin, B. Zappone and S. Lecommandoux, Biomacromolecules, 2005, 6, 2213 CrossRef CAS.
- (a) S. Iijima, Nature, 1991, 354, 56 CrossRef CAS; (b) M. M. J. Treacy, T. W. Ebbesen and J. M. Gibson, Nature, 1996, 381, 678 CrossRef CAS; (c) T. W. Ebbesen, H. J. Lozec, H. Hiura, J. W. Bennett, H. F. Ghaemi and T. Thio, Nature, 1996, 382, 54 CrossRef CAS; (d) R. S. Ruoff and D. C. Lorents, Carbon, 1995, 33, 925 CrossRef CAS.
- (a) J. Chen, M. A. Hamon, H. Hu, Y. Chen, A. M. Rao, P. C. Eklund and R. C. Haddon, Science, 1998, 282, 95 CrossRef CAS; (b) S. H. Lee, J. S. Park, C. M. Koo, B. K. Lim and S. O. Kim, Macromol. Res., 2008, 16, 261 Search PubMed; (c) B. K. Lim, S. H. Lee, J. S. Park and S. O. Kim, Macromol. Res., 2009, 17, 666 Search PubMed; (d) S. H. Lee, J. S. Park, B. K. Lim, C. B. Mo, W. J. Lee, J. M. Lee, S. H. Hong and S. O. Kim, Soft Matter, 2010, 6, 120 RSC.
- (a) K. Balasubramanian and M. Burghard, Small, 2005, 1, 180 CrossRef CAS; (b) I. Dumitrescu, N. R. Wilson and J. V. Macpherson, J. Phys. Chem. C, 2007, 111, 12944 CrossRef CAS.
- (a) R. Czerw, M. Terrones, J.-C. Charlier, X. Blase, B. Foley, R. Kamalakaran, N. Grobert, H. Terrones, D. Tekleab, P. M. Ajayan, W. Blau, M. Rühile and D. L. Carroll, Nano Lett., 2001, 1, 457 CrossRef CAS; (b) M. Terrones, P. M. Ajayan, F. Banhart, X. Blase, D. L. Carroll, J. C. Charlier, R. Czerw, B. Foley, N. Grobert, R. Kamalakaran, P. Kohler-Redlich, M. Rühile, T. Seeger and H. Terrones, Appl. Phys. A: Mater. Sci. Process., 2002, 74, 355 CAS.
- (a) D. H. Lee, D. O. Shin, W. J. Lee and S. O. Kim, Adv. Mater., 2008, 20, 2480 CrossRef CAS; (b) D. H. Lee, W. J. Lee and S. O. Kim, Chem. Mater., 2009, 21, 1368 CrossRef CAS; (c) D. H. Lee, S. O. Kim and W. J. Lee, J. Phys. Chem. C, 2010, 114, 3454 CrossRef CAS; (d) D. H. Lee, J. E. Kim, T. H. Han, J. W. Hwang, S. W. Jeon, S.-Y. Choi, S. H. Hong, W. J. Lee, R. S. Ruoff and S. O. Kim, Adv. Mater., 2010, 22, 1247 CrossRef CAS.
- D. H. Lee, W. J. Lee and S. O. Kim, Nano Lett., 2009, 9, 1427 CrossRef CAS.
- (a) E. A. Whitsitt and A. R. Barron, Nano Lett., 2003, 3, 775 CrossRef CAS; (b) R. Colorado and A. R. Barron, Chem. Mater., 2004, 16, 2691 CrossRef.
- M. Kanugo, H. S. Isaacs and S. S. Wong, J. Phys. Chem. C, 2007, 111, 17730 CrossRef CAS.
- T. Seeger, Th. Köhler, Th. Frauenheim, N. Grobert, M. Rühle, M. Terrones and G. Seifert, Chem. Commun., 2002, 34 RSC.
- (a) R. K. Pai and S. Pillai, Cryst. Growth Des., 2007, 7, 215 CrossRef CAS; (b) R. K. Pai, S. Hild, A. Ziegler and O. Marti, Langmuir, 2004, 20, 3123 CrossRef CAS.
- H. Liu, S. Li, J. Zhai, H. Li, Q. Zheng, L. Jiang and D. Zhu, Angew. Chem., Int. Ed., 2004, 43, 1146 CrossRef CAS.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Experimental details. Fig. S1: TEM images and EDX chemical analyses of (a) bare NCNTs, (b) SiO2 coated NCNTs and (c) CaCO3 coated NCNTs. Fig. S2: TEM images and EDX chemical analyses of (a) SiO2/undoped CNT, (b) SiO2/hydrophilic oxygenated CNT, (c) CaCO3/undoped CNT, and (d) CaCO3/hydrophilic oxygenated CNT. Table S1: Elemental analyses (atom%) using EDX. Fig. S3: (a) Static water contact angles (CA) of undoped CNT, hydrophilic oxygenated CNT, and NCNT, (b) FT-Raman spectrum of undoped CNT and hydrophilic oxygenated CNT, TEM images of (c) SiO2/undoped CNT (left), SiO2/hydrophilic oxygenated CNT (middle), and SiO2/NCNT (right); (d) CaCO3/undoped CNT (left), CaCO3/hydrophilic oxygenated CNT (middle), and CaCO3/NCNT (right). Fig. S4: Individually separated (a) SiO2 coated NCNTs and (b) CaCO3 coated NCNTs. Fig. S5: Comparison between (a) SiO2 half-coated NCNTs and (b) SiO2 fully coated NCNTs. Fig. S6: Proposed biomimetic mineralization mechanisms for (a) SiO2 coated NCNTs, and (b) CaCO3 coated NCNTs at pyridinic nitrogen nucleation sites. See DOI: 10.1039/c0cc04237d |
| This journal is © The Royal Society of Chemistry 2011 |
