Fluorescent and pH-responsive diblock copolymer-coated core–shell CdSe/ZnS particles for a color-displaying, ratiometric pH sensor†
Kwanyeol
Paek
,
Sunhaeng
Chung
,
Chul-Hee
Cho
and
Bumjoon J.
Kim
*
Department of Chemical and Biomolecular Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon 305-701, Korea. E-mail: bumjoonkim@kaist.ac.kr; Fax: +82-42-350-3910; Tel: +82-42-350-3935
First published on 19th August 2011
Abstract
A color distinctive, ratiometric pH sensor was demonstrated using pH responsive and fluorescent (PyMMP-b-P2VP) diblock copolymer coated CdSe/ZnS QDs. Due to the change in the P2VP conformations in response to pH change, the color of QDs in solution changes distinctly from blue to red.
Polymer-coated nanoparticles have received considerable attention because of their potential applications in electronic and optical devices, as surfactants, and in catalysis.1–5 The inorganic core of nanoparticles can provide unique optical, electrical, magnetic, and catalytic properties, and the polymeric (organic) coating can stabilize the nanoparticles by inhibiting their aggregation; these polymers can be independently designed to precisely control the interaction between the nanoparticles and the outside medium. Thus, the appropriate polymeric coating allows the polymer-coated nanoparticles to interact favorably with the polymer and the fluid matrix to produce new morphologies and enhances their bulk properties, e.g., the thermal, electrical and mechanical properties of the composites.6–10 In addition, the use of stimuli-responsive polymers allows the polymer-coated nanoparticles to be used as sensors.11,12
Quantum dots (QDs) have several advantages over the traditional materials used in sensors, including high resistance to photobleaching, high quantum yield, and narrow emission peaks with broad absorption spectra.13 Several research groups have reported QD-based pH sensors consisting of an inorganic core and an amphiphilic organic shell, in which the photoluminescence (PL) intensity of the QDs changed as a function of pH due to PL quenching between the organic molecules and QDs.14–18 However, these sensors, which have a single intensity-based response, have mainly been used as intracellular sensors and not as ratiometric sensors. To date, only few studies have reported the development of a QD-based ratiometric sensor with dual emissions.19,20 However, this sensor typically requires a multistep synthesis and a specific organic molecule, which limits the versatility of the approach. In addition, due to the lack of diversity in the colors emitted by QDs and dyes or unbalanced emission intensities between QDs and dyes, it has not been possible to effectively monitor the external changes based on the direct visual observation of color changes; consequently, an expensive photoluminescence detector has been required.
In this communication, we demonstrate a simple and robust route for the fabrication of a ratiometric pH sensor, based on block copolymer-capped QDs, that exhibits a distinctive color change in response to a change in pH. Cadmium selenide/zinc sulfide (CdSe/ZnS) QDs were carefully chosen and coated with pH responsive and fluorescent poly((1-pyrene)methyl-2-methyl-2-propenoate))-b-poly(2-vinylpyridine) (PyMMP-b-P2VP) block copolymers. Förster resonance energy transfer (FRET) between these two distinguishable chromophores of red emitting CdSe/ZnS cores and blue emitting pyrene shells is governed by the interspacing between them, which can be controlled through manipulation of the conformational features of the P2VP chains that respond to pH changes. Furthermore, the emission intensity ratio between the QDs and pyrene blocks can easily be balanced and controlled by simply tuning the number of repeating pyrene units in the (PyMMP-b-P2VP) chain. Therefore, our polymer-coated QDs can exhibit not only ratiometric pH-dependent fluorescence spectra but also a clear color change from blue to purple to red as a function of pH.
Scheme 1 presents a synthetic procedure for preparing (PyMMP-b-P2VP) coated CdSe/ZnS QDs ((PyMMP-b-P2VP)-QDs). Red-emitting CdSe/ZnS QDs (1) were synthesized by a one-pot synthesis method that used oleic acid for stabilization,21 and then P2VP-b-PyMMP diblock copolymers were synthesized from the QD surface by reversible addition–fragmentation chain transfer (RAFT) polymerization using the “grafting-from” method. In this process, oleic acid ligands on the QD surface were first replaced with the thiol-terminated trithiocarbonate RAFT chain transfer agent (3) via ligand exchange. Compared to the previously reported phosphine oxide-functionalized RAFT chain transfer agent,22 our thiol-terminated RAFT agent can be synthesized easily using only a two-step reaction and can be applied to other nanoparticle systems, including gold and silver, because of strong binding interactions between the thiol group and the nanoparticle surface. Because the molecular weight (Mn) of the P2VP block is a critical parameter that determines the distance between the two chromophores and the Mn of the PyMMP block can influence the brightness of blue emitters, the Mn of both the P2VP and PyMMP blocks were carefully controlled. The total Mn of P2VP-b-PyMMP was 4.7 kg mol−1 with a polydispersity index (PDI) of 1.17. The Mn and PDI of the P2VP block were 4.0 kg mol−1 and 1.10, respectively. Therefore, the radius of gyration (Rg) of P2VP was calculated to be 1.7 nm, approximately corresponding to the distance between the QDs and the PyMMP block when P2VP is not swelled by solvent. The areal chain density (Σ) of polymers on the QD surface was calculated to be 2.5 chains per nm2 based on the QD core size, which was determined using TEM, and the weight fraction of the QD core and polymer ligands in the (PyMMP-b-P2VP)-QDs, which was determined using thermogravimetric analysis (TGA).23
 | ||
| Scheme 1 Synthesis of (PyMMP-b-P2VP)-CdSe/ZnS QDs. | ||
Fig. 1 shows representative TEM images of the CdSe/ZnS QDs before and after polymerization. The core diameters of three different QDs were all approximately 8.7 nm, indicating that the particle size was not affected by polymerization. Oleic acid-stabilized QDs were well dispersed in organic solvents, such as chloroform, hexane, toluene and THF, but they could not be dispersed in alcohol. Conversely, the (PyMMP-b-P2VP)-QDs could easily be dispersed in alcohol. This result indicated that PyMMP-b-P2VP was successfully polymerized on the CdSe/ZnS QD surface via the grafting-from method.
 | ||
| Fig. 1 TEM images of (a) OA-coated CdSe/ZnS, (b) P2VP-coated CdSe/ZnS, and (c) (PyMMP-b-P2VP) coated CdSe/ZnS. Scale bar is 50 nm. | ||
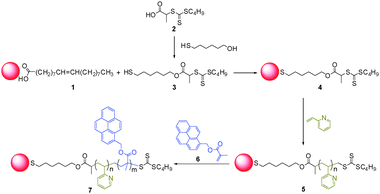
Fig. 2 shows photographic images and the PL spectra of the (PyMMP-b-P2VP)-QDs in buffer solution with a small amount of DMF (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
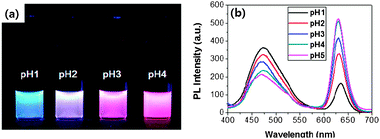
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) at different pH conditions. The concentration of (PyMMP-b-P2VP)-QDs in each solution was maintained at 0.1 mg ml−1. The color of the (PyMMP-b-P2VP)-QD solution dramatically changed from blue to purple to red as the pH increased. These colors matched well with the corresponding PL spectra shown in Fig. 2(b). The PL spectra of the (PyMMP-b-P2VP)-QDs exhibit two emission peaks due to the PyMMP blocks and the CdSe/ZnS QDs at 470 nm and 630 nm, respectively. The paired pyrenes have been shown to exhibit a single PL band at 470 nm because of their excimer formation.24 The PL intensity at 470 nm gradually decreases as the pH of the solution increases from pH 1 to pH 4. In contrast, the PL intensity at 630 nm increases as the pH of the buffer solution increases. At pH 5, the peak reaches saturation, and the solution exhibits no further change in color. The color and PL changes as a function of pH can be correlated to a change in the P2VP chain conformation at different pH conditions. Because the pKa of P2VP is around 3,25 the P2VP chains were protonated and swelled by water below pH 3. Under these conditions, the distance between the two chromophores, i.e., the blue-emitting PyMMP block and the red-emitting QDs, becomes significantly larger and suppresses the FRET from the higher energy PyMMP to the lower energy QDs. The FRET efficiency is inversely proportional to the sixth power of the FRET donor-to-acceptor separation distance because of a dipole–dipole coupling mechanism.26 Conversely, the P2VP chains were deprotonated and collapsed at a higher pH, thus facilitating the FRET from the PyMMP molecules to the QDs and causing the observed color change from blue to red. For further quantitative analysis, the PL intensity ratio at different emission levels of 630 nm and 470 nm (I630 nm/I470 nm) was plotted as a function of pH, as shown in Fig. 3(a). Interestingly, the plot of pH versus I630 nm/I470 nm was almost linear in the pH range of 1–4, indicating that the response is ratiometric in this range. Subsequently, the plot became saturated in the pH range of 4–5.
1 v/v) at different pH conditions. The concentration of (PyMMP-b-P2VP)-QDs in each solution was maintained at 0.1 mg ml−1. The color of the (PyMMP-b-P2VP)-QD solution dramatically changed from blue to purple to red as the pH increased. These colors matched well with the corresponding PL spectra shown in Fig. 2(b). The PL spectra of the (PyMMP-b-P2VP)-QDs exhibit two emission peaks due to the PyMMP blocks and the CdSe/ZnS QDs at 470 nm and 630 nm, respectively. The paired pyrenes have been shown to exhibit a single PL band at 470 nm because of their excimer formation.24 The PL intensity at 470 nm gradually decreases as the pH of the solution increases from pH 1 to pH 4. In contrast, the PL intensity at 630 nm increases as the pH of the buffer solution increases. At pH 5, the peak reaches saturation, and the solution exhibits no further change in color. The color and PL changes as a function of pH can be correlated to a change in the P2VP chain conformation at different pH conditions. Because the pKa of P2VP is around 3,25 the P2VP chains were protonated and swelled by water below pH 3. Under these conditions, the distance between the two chromophores, i.e., the blue-emitting PyMMP block and the red-emitting QDs, becomes significantly larger and suppresses the FRET from the higher energy PyMMP to the lower energy QDs. The FRET efficiency is inversely proportional to the sixth power of the FRET donor-to-acceptor separation distance because of a dipole–dipole coupling mechanism.26 Conversely, the P2VP chains were deprotonated and collapsed at a higher pH, thus facilitating the FRET from the PyMMP molecules to the QDs and causing the observed color change from blue to red. For further quantitative analysis, the PL intensity ratio at different emission levels of 630 nm and 470 nm (I630 nm/I470 nm) was plotted as a function of pH, as shown in Fig. 3(a). Interestingly, the plot of pH versus I630 nm/I470 nm was almost linear in the pH range of 1–4, indicating that the response is ratiometric in this range. Subsequently, the plot became saturated in the pH range of 4–5.
 | ||
| Fig. 2 (a) Photographic image of (PyMMP-b-P2VP)-QDs solutions under irradiation at 365 nm using a UV lamp, and (b) PL spectra of (PyMMP-b-P2VP)-QDs. | ||
 | ||
| Fig. 3 (a) PL intensity changes (I630/I470) as a function of pH, and (b) hydrodynamic size of (PyMMP-b-P2VP)-QDs as a function of pH, measured by DLS. | ||
A deeper insight into the dramatic change in PL and color spectra of (PyMMP-b-P2VP)-QDs as a function of pH can be gleaned by examining the change in particle size viadynamic light scattering (DLS) measurements (Fig. 3(b)). The hydrodynamic sizes of the (PyMMP-b-P2VP)-QD particles at different pH conditions were measured; the PL change of the particles was found to correlate to the distance between the PyMMP and QD core chromophores. The distance can be estimated by measuring the particle size at a given pH. The particle size was constant at 12 nm above pH 4, showing good agreement with the sum of the QD core (∼8.7 nm) and twice the Rg of the P2VP chains (∼1.7 nm). Under these pH conditions, the distance between the PyMMP and QD chromophores is short enough to induce a strong increase in FRET efficiency from the PyMMP block to the QDs. As the pH decreases, the particle size gradually increases from 13 nm (pH 3) to 19 nm (pH 2) to 36 nm (pH 1). Because the hydrodynamic particle size at low pH can be overestimated by DLS measurements due to extensive swelling of the polymers by the solvent,27 the increase in the particle size is not equal to the increase in the donor-to-acceptor distance. However, it is worthwhile to note that the trend of the particle size dependence on the change in pH is consistent with that of the P2VP conformation and the PL spectra shown in Fig. 2. As the pH decreases from pH 4 to pH 1, the P2VP chains were swelled by water, increasing the particle size and the donor-to-acceptor distance. The corresponding decrease in the FRET efficiency results in an increase in the PL intensity of PyMMP, which is a FRET donor, and a decrease in the PL intensity of the QD, which is a FRET acceptor. Conversely, the P2VP blocks were fully deprotonated above pH 4, and the corresponding particle size was unchanged. As a result, there is no further change in the PL intensities of the two different chromophores.
Further evidence for FRET is provided by the PL spectra of P2VP-coated QDs under different pH conditions (Fig. S5, ESI†). These P2VP-coated CdSe/ZnS QDs are identical to the (PyMMP-b-P2VP)-QDs, but the PyMMP block is not included in the polymer shell. The PL intensity of the P2VP-coated QDs was measured as a function of pH, and a single emission peak at 630 nm was observed which originated from the CdSe/ZnS QDs. In addition, the PL intensity at 630 nm did not change in the range of pH 1–4. These results indicate that the change in the PL intensity at 630 nm at a lower pH is not caused by degradation of the QDs. In addition, it is known that the photoluminescence property of pyrene is barely influenced by the change in pH.28 Therefore, FRET is mainly responsible for the observed changes in PL intensity, which results in a pH ratiometric response in the PL intensity of the (PyMMP-b-P2VP)-QDs.
In conclusion, we have shown that a ratiometric pH sensor with a color indicator can be constructed using the FRET process. Fluorescent and pH-responsive PyMMP-b-P2VP diblock copolymers were designed and synthesized on the CdSe/ZnS QDs using the facile “grafting-from” method with RAFT polymerization. The emission intensity ratio between the QDs and pyrene blocks can be balanced and controlled by simply tuning the repeating pyrene units. Red and blue colors are emitted from the CdSe/ZnS cores and pyrene shells, respectively, and the FRET efficiency between these two distinguishable chromophores is governed by the interspacing between them, which can easily be controlled through manipulation of the conformational features of the P2VP chains that respond to changes in pH. Interestingly, the trend of the particle size dependence on the change in pH, based on DLS measurements, is consistent with that of P2VP conformation and PL spectra, indicating that the ratiometric behavior of our sensor is the result of the P2VP conformation change and the corresponding change in FRET efficiency when the pH changes. In addition, (PyMMP-b-P2VP)-QDs exhibited good stability under a wide range of pH conditions. At an optimal concentration of QDs, high brightness and sensitivity can also be obtained. Our approach of diblock copolymer-coated QDs provided a simple and robust route for the construction of a ratiometric pH sensor, which can be easily extended to other types of sensors for the detection of alternate stimuli, such as temperature and light, using appropriate, responsive polymers.
This research was supported by the Korea Research Foundation Grant, funded by the Korean Government (2009-0088551, 2010-0029611, 2010-0011033). The authors thank Prof. Ryan C. Hayward for useful discussions.
Notes and references
- T. F. Jaramillo, S. H. Baeck, B. R. Cuenya and E. W. McFarland, J. Am. Chem. Soc., 2003, 125, 7148–7149 CrossRef CAS.
- B. J. Kim, G. H. Fredrickson, C. J. Hawker and E. J. Kramer, Langmuir, 2007, 23, 7804–7809 CrossRef CAS.
- S. Lee, B. Lee, B. J. Kim, J. Park, M. Yoo, W. K. Bae, K. Char, C. J. Hawker, J. Bang and J. H. Cho, J. Am. Chem. Soc., 2009, 131, 2579–2587 CrossRef CAS.
- B. J. Kim, G. H. Fredrickson, J. Bang, C. J. Hawker and E. J. Kramer, Macromolecules, 2009, 42, 6193–6201 CrossRef CAS.
- S. G. Jang, A. Khan, M. D. Dimitriou, B. J. Kim, N. A. Lynd, E. J. Kramer and C. J. Hawker, Soft Matter, 2011, 7, 6255–6263 RSC.
- H. Chung, K. Ohno, T. Fukuda and R. J. Composto, Nano Lett., 2005, 5, 1878–1882 CrossRef CAS.
- K. Stratford, R. Adhikari, I. Pagonabarraga, J. C. Desplat and M. E. Cates, Science, 2005, 309, 2198–2201 CrossRef CAS.
- T. Kwon, M. Min, H. Lee and B. J. Kim, J. Mater. Chem., 2011, 21, 11956–11960 RSC.
- M. Yoo, S. Kim, J. Lim, E. J. Kramer, C. J. Hawker, B. J. Kim and J. Bang, Macromolecules, 2010, 43, 3570–3575 CrossRef CAS.
- J. Lim, H. Yang, K. Paek, C. Cho, S. Kim, J. Bang and B. J. Kim, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 3464–3474 CrossRef CAS.
- M. Q. Zhu, L. Q. Wang, G. J. Exarhos and A. D. Q. Li, J. Am. Chem. Soc., 2004, 126, 2656–2657 CrossRef CAS.
- O. J. Cayre, N. Chagneux and S. Biggs, Soft Matter, 2011, 7, 2211–2234 RSC.
- A. P. Alivisatos, Science, 1996, 271, 933–937 CAS.
- M. F. Frasco and N. Chaniotakis, Sensors, 2009, 9, 7266–7286 CrossRef CAS.
- Y. S. Liu, Y. H. Sun, P. T. Vernier, C. H. Liang, S. Y. C. Chong and M. A. Gundersen, J. Phys. Chem. C, 2007, 111, 2872–2878 CAS.
- A. S. Susha, A. M. Javier, W. J. Parak and A. L. Rogach, Colloids Surf., A, 2006, 281, 40–43 CrossRef CAS.
- M. Suzuki, Y. Husimi, H. Komatsu, K. Suzuki and K. T. Douglas, J. Am. Chem. Soc., 2008, 130, 5720–5725 CrossRef CAS.
- M. Tomasulo, I. Yildiz and F. M. Raymo, J. Phys. Chem. B, 2006, 110, 3853–3855 CrossRef CAS.
- T. Jin, A. Sasaki, M. Kinjo and J. Miyazaki, Chem. Commun., 2010, 46, 2408–2410 RSC.
- P. T. Snee, R. C. Somers, G. Nair, J. P. Zimmer, M. G. Bawendi and D. G. Nocera, J. Am. Chem. Soc., 2006, 128, 13320–13321 CrossRef CAS.
- W. K. Bae, K. Char, H. Hur and S. Lee, Chem. Mater., 2008, 20, 531–539 CrossRef CAS.
- H. Skaff and T. Emrick, Angew. Chem., Int. Ed., 2004, 43, 5383–5386 CrossRef CAS.
- B. J. Kim, J. Bang, C. J. Hawker and E. J. Kramer, Macromolecules, 2006, 39, 4108–4114 CrossRef CAS.
- T. Forster, Angew. Chem., Int. Ed., 1969, 8, 333–343 CrossRef.
- R. C. Hayward, B. F. Chmelka and E. J. Kramer, Adv. Mater., 2005, 17, 2591–2595 CrossRef CAS.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Plenum, New York, 2nd edn, 1999 Search PubMed.
- S. R. Marek, C. A. Conn and N. A. Peppas, Polymer, 2010, 51, 1237–1243 CrossRef CAS.
- S. W. Hong, K. H. Kim, J. Huh, C. H. Ahn and W. H. Jo, Chem. Mater., 2005, 17, 6213–6215 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and additional data (Schemes S1 and S2, and Fig. S1–S5). See DOI: 10.1039/c1cc13848k |
| This journal is © The Royal Society of Chemistry 2011 |
