Full quantification of selenium species by RP and AF-ICP-qMS with on-line isotope dilution in serum samples from mercury-exposed people supplemented with selenium-enriched yeast†
Yu-Feng
Li
a,
Liang
Hu
a,
Bai
Li
a,
Xiaohan
Huang
a,
Erik H.
Larsen
c,
Yuxi
Gao
a,
Zhifang
Chai
a and
Chunying
Chen
*ab
aCAS Key Laboratory of Nuclear Analytical Techniques and CAS Key Laboratory for Biological Effects of Nanomaterials and Nanosafety, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing, 100049, China. E-mail: chenchy@nanoctr.cn; Fax: +86-10-8823 3195; Tel: +86-10-8254 5560
bNational Center for Nanoscience and Technology, Beijing, 100190, China
cNational Food Institute, Technical University of Denmark, Copenhagen, DK-1790, Denmark
First published on 25th November 2010
Abstract
Accurate determination of selenium (Se) species in biological samples is a critical issue because Se commonly occurs at low levels and in diverse species. The method for the full quantification of Se species in serum samples was proposed through combined ion-pair reverse-phase (RP) chromatography and affinity chromatography (AF) hyphenated to inductively coupled plasma-(quadrupole) mass spectrometry (ICP-qMS) with post-column isotope dilution analysis (IDA) and a collision cell technique (CCT). Different Se species like inorganic Se (Se4+ and Se6+), selenocystine (SeCys), selenomethionine (SeMet), selenoprotein P (SelP), selenoalbumin (SeAlb) and glutathione peroxidase (GPx) can be separated and quantified. The proposed methodology was used to qualitatively and quantitatively study the dynamic distribution of Se species in human serum samples from the Hg-contaminated area after supplementation with 100 μg of Se daily as Se-enriched yeast for 180 days. SelP takes up almost half and even more of the total Se and increases with the Se administration. The repeatability in terms of relative standard deviation (R.S.D. %, n = 10) is 6% for GPx and SelP and 5% for SeAlb. The detection limits are 0.1 μg Se L−1 for GPx and other non-retained Se compounds, 1.0 μg Se L−1 for SelP and 1.2 μg Se L−1 for SeAlb, 1.3 μg Se L−1 for inorganic Se; 1.2 μg Se L−1 for SeCys; 1.1 μg Se L−1 for SeMet, respectively.
Introduction
Selenium (Se) is an essential micronutrient because of its unique antioxidant properties and its ability to regulate thyroid gland metabolism.1As a well-known highly hazardous element, mercury (Hg) can bring toxic effects to the immune system, kidneys, lungs, and nervous tissues, and is linked with a number of human health diseases.2 The town of Wanshan in Guizhou was once the major Hg-mining area in China, where large-scale production of Hg lasted for more than 50 years before closure in 2002. Owing to the natural and anthropogenic factors, people living in this area suffered elevated Hg exposure and increased oxidative damage.3–5
A Se supplementation trial in persons exposed to Hg through fish consumption (in which most Hg is in an organic form as methylmercury) found reduced pubic hair Hg level,6 which suggested the reduced accumulation of Hg after Se supplementation. However, to the best of our knowledge, there is no investigation on the effects of Se supplementation in long-term Hg-exposed populations living in Hg-mining area, who are exposed mostly to inorganic and elemental Hg. Therefore, a study was initiated to evaluate the effects of Se supplementation in Hg-exposed volunteers from Wanshan Hg-mining area. Hair, urine and blood samples were collected. Increased excretion of Hg was found after Se supplementation and the major Hg and Se species were characterized in the urine samples.7 However, no correlation between Hg and Se was found in these urine samples although the coaccumulation of Se and Hg in human and other mammalian organisms had been long found.8 As the extension of this work, the coaccumulation of Se and Hg in human serum samples will be further studied. In this study, however, only the Se species in serum samples before and after Se supplementation in these volunteers will be studied.
Se is mainly incorporated into three proteins in serum, i.e. glutathione peroxidase (GPx), selenoprotein P (SelP) and albumin (SeAlb), which can be separated by anion exchange chromatography (AEC),9,10 size exclusion chromatography (SEC)11 or affinity chromatography (AC).9,12,13 However, quantitative study could only find about 90.7%10 and 85.9–92.5%9 of total Se in serum. About 10% of total Se in serum is still unidentified.
Information on the presence of selenoamionacids and inorganic Se in serum is scarce. Previous work has established and optimized analytical methods to identify and quantify selenoaminoacids and inorganic Se in biological samples by reverse-phase chromatography (RP).7,14–16 To our knowledge, there were few articles to qualitatively and quantitatively determine both small molecular selenocompounds and Se-containing proteins in human serum samples. Therefore, in this study we proposed a method by the combination of RP and AF chromatography coupled to ICP-MS. Through the proposed method, Se-containing proteins (SelP, SeAlb, GPx), and small selenocompounds like SeCys, SeMet, inorganic Se in serum in Hg-exposed people after Se supplementation can be separated and quantified by RP and AF-ICP-qMS with post-column isotope dilution analysis (IDA) and a collision cell technique (CCT). The dynamic distribution of Se species in human serum samples will also be studied.
Experimental
Instrument
A Symmertryshield RP18 column (150 × 3.9 mm, Waters, Milford, USA) and affinity “HiTrap-heparin” sepharose (1 mL) and “HiTrap-blue” sepharose (1 mL) columns (Pharmacia, Uppsala, Sweden) were employed for the separation of the small molecular Se compounds and selenocontaining proteins. The columns were connected to a HPLC system consisting of a liquid chromatography pump (WAT055028 metal-free 626 pump, Waters, Milford, USA). A six-way Rheodyne valve (Model 5012) was also used for affinity chromatography. The continuous addition of the spike was performed by a peristaltic pump (Model HP4, Scharlau Science, Barcelona, Spain). Samples were loaded with a syringe into a 100 μL sample loop. All separations were performed at room temperature. UV measurements were performed with a Waters 484 UV/VIS absorption detector (Waters Corporation, MA, USA). All solutions were prepared with Milli-Q water (18.2 MΩ cm), and filtrated with a 0.22 μm microporous membrane. The column was connected directly to the nebulizer of the ICP-MS system with polyether ether ketone (PEEK) tubing (0.13 mm i.d.). The signal of ICP-MS was triggered by the manual injector of the HPLC. A Thermo Elemental X7 ICP-MS (Thermo Electron Co., USA) was used as the element detector. Optimization was carried out daily with a normal tuning solution (1 ng mL−1, Be, Co, In, U). Raw data were collected by the PlasmaLab software through a personal computer. The peak areas of elemental signals of ICP-MS were used for quantitative analysis online. A continuous flow of 7.5 mL min−1 of hydrogen and helium was introduced into the collision cell as reaction gas. The sample introduction system consisted of a classical Meinhard nebuliser with a Scott double-pass quartz spray chamber cooled down to 2 °C. The collision cell technique (CCT) was employed for the elimination of the polyatomic interferences of 40Ar40Ar and others in the detection of 80Se. The ICP-MS and the HPLC operating conditions used in this work are summarized in Table 1.| Plasma parameters | |
| Power | 1350 W |
| Plasma Ar flow rate | 13 L min−1 |
| Carrier Ar flow rate | 0.75 L min−1 |
| Nebulization Ar rate | 0.84 L min−1 |
| CCT gas and the flow rate | 92.72% (He) + 7.29% (H2),7.5 mL min−1 |
| Isotopes monitored | Se: 76, 77, 78, 80, 82 |
| Dwell time for each isotope | 10 ms |
| Acquisition mode | Time resolved analysis |
| Reverse-phase ion-pair HPLC Conditions | |
| Column | Symmertryshield RP18, 150 × 3.9 mm |
| Mobile phase A | 0.3% CH3OH + 0.1% HFBA |
| Mobile phase B | 5% CH3OH + 0.1% HFBA |
| Gradient program | 0–4 min, mobile phase A, 4–12 min, mobile phase B |
| Flow rate | 1.0 mL min−1 |
| Injection volume | 0.1 mL |
| Pressure | 1700 Psi |
| Affinity column HPLC Conditions | |
| Columns | Hitrap heparin-sepharose (1 mL), Hitrap blue-sepharose (1 mL) |
| Injection volume | 0.1 mL |
| Flow rate | 0.8 L min−1 |
| Pressure | 250 Psi |
| Mobile phase A | Binding buffer, ammonium acetate, 0.05 mol mL−1, pH 7 |
| Mobile phase B | Elution buffer, ammonium acetate, 1.5 mol mL−1, pH 7 |
| Gradient program | 0–5 min, position 1 (P1), mobile phase A; 5–10 min, position 2 (P2), mobile phase B; 10–15 min, position 1(P1), mobile phase B; 15–20 min, position 1(P1), mobile phase A |
Reagents and standards
All reagents were of analytical reagent grade. Heptafluorobutanoic acid (HFBA) was purchased from Fluka (Switzerland), while Triton X-100 from Amersco (USA). Ammonium acetate and nitric acid were obtained from Beihua (China), while methanol from Guangfu (China). Enriched 78Se was obtained from Cambridge Isotope Laboratories (USA).Stock standard solutions of 100 mg Se L−1 were prepared in deionized water from selenocysteine (Sigma, St. Louis, USA), selenomethionine (Sigma, St. Louis, USA), sodium selenate (Na2SeO4, Alfa Aesar), sodium selenite (Na2SeO3, Zhonglian, China). The working standard solutions were prepared in Milli-Q water by dilution of stock solutions as required daily and stored in the dark.
Collection and preparation of serum samples
Whole blood samples were collected from 53 volunteers from Wanshan Hg-mining area who had been supplemented with 100 μg of Se per day from day 0 to 180 in the form of Se-enriched yeast (SelenoPrecise, Pharma Nord, Denmark). The blood samples were collected on day 0 to day 30, 60, 90, 120, 150, 180 and 360, where day 0 was the day before the supplementation and day 30 to 60, 90, 120, 150, 180, and 360 were the corresponding days after supplementation. The blood samples of at least 5 mL were collected in the morning after overnight fasting. The serum samples were obtained by centrifugation (10 min at 1500 g) 2–4 h after blood collection and stored at −20 °C until analysis. Serum samples were analyzed within a week after the collection.Serum samples were simply 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 diluted with 0.05 mol L−1 ammonium acetate (pH = 7) and centrifuged at 10000 g for 30 min by centrifugal filtration tube (molecular weight cut off at 3000 Da). Supernatant (molecular weight > 3000 Da) is prepared for quantitative analysis of GPx, SelP and SeAlb, while lower-layer liquid (molecular weight < 3000 Da) is used for quantitative analysis of Se4+ and Se6+ (inorganic Se), SeCys, and SeMet. Prior to injection, all samples were filtrated with 0.45 μm microporous membrane.
4 diluted with 0.05 mol L−1 ammonium acetate (pH = 7) and centrifuged at 10000 g for 30 min by centrifugal filtration tube (molecular weight cut off at 3000 Da). Supernatant (molecular weight > 3000 Da) is prepared for quantitative analysis of GPx, SelP and SeAlb, while lower-layer liquid (molecular weight < 3000 Da) is used for quantitative analysis of Se4+ and Se6+ (inorganic Se), SeCys, and SeMet. Prior to injection, all samples were filtrated with 0.45 μm microporous membrane.
Analytical procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 with solution (0.1% HNO3 + 0.1% HFBA + 0.1% Triton X-100) were directly introduced into ICP-qMS by 2% (v/v) HNO3. The recovery was 97% by using human serum reference materials BCR-637 from our experiments. The ICP-MS condition, which was the same as Se speciation, shown in Table 1.
4 with solution (0.1% HNO3 + 0.1% HFBA + 0.1% Triton X-100) were directly introduced into ICP-qMS by 2% (v/v) HNO3. The recovery was 97% by using human serum reference materials BCR-637 from our experiments. The ICP-MS condition, which was the same as Se speciation, shown in Table 1.
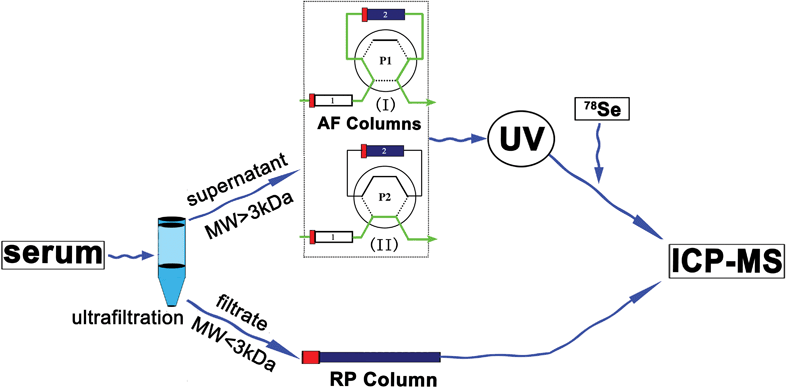 | ||
| Fig. 1 Scheme of Se species analysis in human serum including small Se compounds and Se-containing proteins by ion-pair reverse-phase chromatography and affinity chromatography coupled to post-column isotope dilution analysis ICP-MS, where in the AF columns, column 1 is heparin sepharose, column 2 is blue-sepharose. | ||
In Fig. 1, the heparin-sepharose column 1 can selectively retain SelP, while the blue sepharose 2 can retain both SelP and albumin. The serum samples first passed through both column 1 and 2 using mobile phase A, where SelP was retained in column 1 and SeAlb in column 2 at position 1 (P1). GPx and other non-retained Se compounds were first eluted since they lack affinity for heparin or blue-sepharose. Then mobile phase B was used to elute SelP at position 2 (P2). The SeAlb was eluted using mobile phase B at P1, then mobile phase A was used at P1 for the preparation of the next injection. The outlet of the chromatographic column was connected through a T-piece where an enriched 78Se spike was continuously added with a flow rate of 0.8 mL min−1 and the mixture was nebulized into the plasma for the measurement of the Se in binding proteins in human serum.
Results and discussion
Separation and quantification of small molecular selenocompounds
As is known to all, the pH value and methanol concentration in the mobile phase and the concentrations of ion-pair reagents can affect the separation efficiency of small molecular selenocompounds. Here, chromatographic parameters, such as the concentrations of HFBA and methanol was further changed and optimized. When mobile phase contained 2% CH3OH and 0.1% HFBA, peaks obtained from aqueous SeMet standard was broader and the elution time was longer than that using the mobile phase containing 5% CH3OH and 0.3% HFBA. Consequently, the later was also used to reduce the elution time of SeMet.The chromatogram of the small molecular Se compounds is shown in Fig. 2. It is hard to separate Se4+ and Se6+. However, the separation of Se4+ and Se6+ in serum samples can be achieved by using anion exchange column that was reported in our other paper.21 We found that Se4+ is the major inorganic Se forms but only accounts for 1.8% to 2.6% of total Se.
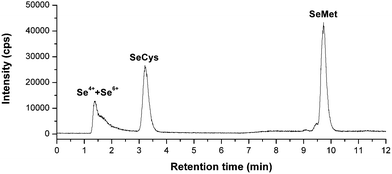 | ||
| Fig. 2 Chromatogram of the small molecular Se compounds in a serum sample. Column: Symmertryshield RP18, 150 × 3.9 mm, mobile phase: A, 0.3% CH3OH + 0.1% HFBA, B, 5% CH3OH + 0.3% HFBA, 0–4 min, A, 4–12 min, B, flow rate: 1 mL min−1, injection volume: 0.1 mL. | ||
Calibration curves based on peak area were linear with correlation coefficients (r2), which is better than 0.9992 for each species in the range studied (1–100 ng mL−1). The detection limits were estimated based on the concentration (as element) necessary to yield a net signal equal to three times the standard deviation of the background. The IP-RP-HPLC method detection limits (as element) were 1.3, 1.2, and 1.1 ng mL−1 for Se4+ and Se6+, SeCys, SeMet, respectively.
Quantification of SelP, SeAlb, GPx and other non-retained Se compounds by AF-IDA-ICP-qMS
Fig. 3 shows the separation and isotope dilution analysis of Se-containing proteins in serum. It is well known for isotope ratio accurate measurements, every intensity value in the chromatogram had to be corrected for the dead time, which was 35 ns using the usual correction equation.9,22,23 After dead time and mass bias correction, the corrected final mass flow chromatograms in Fig. 3(b) were evaluated from the ratios of 80Se/78Se in Fig. 3(a) using the online isotope equation.20 The intensity of a protein was monitored by UV at 254 nm online as shown in Fig. 3(c). The application of ion-guiding multipole collision cells in ICP-MS using helium as a buffer and hydrogen as a reaction gas is an effective means of reducing or even suppressing argon induced polyatomic ion interferences, 40Ar40Ar and 38Ar40Ar, on the main Se isotopes 80Se and 78Se, respectively.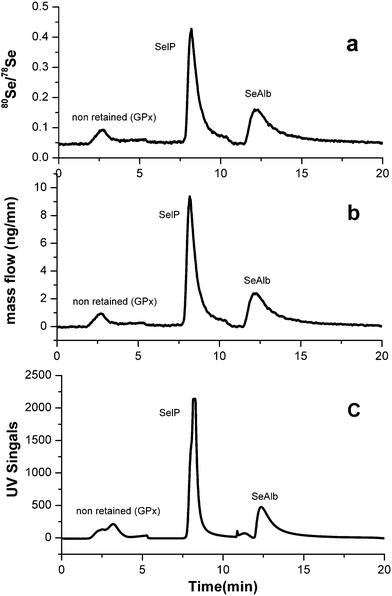 | ||
| Fig. 3 (a), Corresponding Se isotope ratio chromatograms for 80Se/78Se after mathematical corrections. (b), Mass flow chromatogram obtained for 80Se/78Se isotope ratios in a serum sample by AF-HPLC. (c), Corresponding UV signal (λ = 254) of GPx and non-retained Se compounds, SelP, SeAlb in a serum sample. | ||
The use of affinity chromatography by a heparin-sepharose column coupled to a reactive blue-sepharose column allows for a rapid, precise and convenient fractionation of the three main Se-containing proteins in human serum (GPx, SelP and SeAlb). The repeatability in terms of relative standard deviation (R.S.D. %, n = 10) is 6% for GPx and SelP and 5% for SeAlb. The detection limits are also calculated according to the 3s criterion. LODs are 0.1 μg Se L−1 for GPx and other non-retained Se compounds, 1.0 μg Se L−1 for SelP and 1.2 μg Se L−1 for SeAlb, respectively.
Full quantification of both small molecular selenocompounds and selenoproteins in serum samples from one subject
The quantitative results of inorganic Se, SeCys, SeMet, GPx, SelP and SeAlb in serum samples from one subject before and after Se supplementation through the combined application of RP and AF columns hyphenated to ICP-MS are shown in Fig. 4. The undetected Se species was calculated as the total Se contents minus all the detected Se species.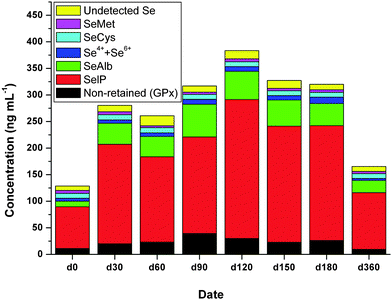 | ||
| Fig. 4 Time-dependent concentrations of Se species in serum samples of one of the Hg-exposed subjects supplemented with Se enriched yeast. Data represent the average values of three independent determinations. | ||
It was found that the total Se significantly increased after Se supplementation by day 0 to day 180, whereas it dropped after the cease of Se supplementation on day 360. It should be noted that the Se level in serum after Se supplementation (d360) are still higher than that before Se supplementation (d0) even after 6 months, which suggest organic Se (Se-enriched yeast in our case) supplementation is efficient to increase serum Se concentrations and this is in agreement with other studies.24
The distribution of Se in different Se species in serum samples from one subject on different days is shown in Table 2. On day 0, Se in SelP, SeAlb and GPx accounts for 61%, 8.1%, 8.7% of total Se, where the distribution of SelP and SeAlb are almost the same as other study but the GPx is less than other study.9 In the study by Reyes et al., the GPx peak stands also for the non-retained Se compounds, which may be the small molecular Se compounds in our study. Therefore, if the small molecular Se compounds are considered, which accounts for 15.7% of total Se, our results also agree well with their finding.9
| Date | Non-retained (GPx) | SelP | SeAlb | Se4+ + Se6+ | SeCys | SeMet | Undetected Se |
|---|---|---|---|---|---|---|---|
| Day 0 | 8.7 | 61.0 | 8.1 | 4.4 | 7.1 | 4.2 | 6.5 |
| Day 30 | 7.2 | 66.7 | 14.0 | 2.2 | 3.6 | 1.9 | 4.3 |
| Day 60 | 8.9 | 61.5 | 14.6 | 2.7 | 3.7 | 1.4 | 7.3 |
| Day 90 | 12.4 | 57.3 | 19.4 | 3.0 | 2.9 | 1.3 | 3.7 |
| Day 120 | 7.9 | 68.1 | 13.9 | 2.3 | 2.4 | 1.4 | 4.0 |
| Day 150 | 7.0 | 66.8 | 15.0 | 2.5 | 2.8 | 1.4 | 4.6 |
| Day 180 | 8.2 | 67.4 | 13.0 | 3.7 | 2.9 | 1.5 | 3.2 |
| Day 360 | 5.8 | 64.4 | 13.8 | 2.3 | 5.5 | 2.5 | 5.6 |
On day 0, trace amount of Se in inorganic Se, SeCys and SeMet in serum sample were found, which accounts for 4.4%, 7.1% and 4.2% of total Se, respectively. Using SEC, Palacios et al.11 did not find such Se molecules, however, this may be attributed to the poor resolving power of SEC. In our study, the undetected Se on d0 through our method accounts for 6.5% of total Se, which suggests other Se species exist.
After Se supplementation, the distribution of Se in SelP and SeAlb increased from 61.0% to 64.6% and 8.1% to 14.9%, respectively, while for the inorganic Se, SeCys and SeMet, it decreased from 4.4% to 2.7%, 7.1% to 3.1% and 4.2% to 1.5%, respectively. The distribution of Se in GPx did not change too much, which is 8.7% and 8.6% before and after Se supplementation. Our results find that most of the supplemented Se went to SeAlb, which is in agreement with the findings by Palacios et al.11
On day 360, when the Se supplementation ceased for 6 months, the Se distribution in SelP and SeAlb is still higher than that on day 0, while for GPx and other non-retained Se compounds, inorganic Se, SeCys and SeMet, it is lower than that on day 0. This may suggest that SelP and SeAlb may serve as the Se pool in the body while GPx etc. can serve as the indicator of Se intake in the body.
Organic Se is more efficient at increasing serum Se concentrations but not necessarily increasing Se status such as GPx. The findings of this study show that the concentration of Se species will reach the top after Se supplementation, i.e., Se in GPx, SelP and SeAlb reach the maximum distribution on day 90, day 120 and day 90, respectively, but the distribution did not change too much in the supplementation phase. It can therefore be concluded that long-term Se supplementation is suitable to increase and thus improve the body Se pools.
Generally, Se can antagonize the toxicity of Hg in mammals, which is presumably attributed to the formation of biologically inert Se–Hg compounds.25 An attempt to search for the possible inert Se–Hg compounds in the serum samples is still under way in our lab.
Conclusions
Analysis of Se species (including Se4+ + Se6+, SeCys, SeMet, GPx, SelP, and SeAlb) in human serum samples is proposed by HPLC-ICP-qMS through ion-pair reversed phase chromatography and affinity chromatography with on-line isotope dilution.The present methodology was applied to study the dynamic distribution of Se in serum samples in long-term Hg exposed people before and after Se supplementation. Se in SelP, SeAlb and GPx accounts for over 77% of total Se while Se in inorganic Se, SeCys and SeMet accounts for about 15% of total Se before Se supplementation. After Se supplementation, the distribution of Se in SelP and SeAlb increased, while decreasing for the inorganic Se, SeCys and SeMet. The distribution of Se in GPx did not change too much before and after Se supplementation. Our results indicate that most of the supplemented Se are bound to SeAlb. Long-term Se supplementation is suitable to increase and thus improve the body Se pools even in Hg-exposed people.
Acknowledgements
This work is supported by NSFC/RGC Joint Research Scheme (20931160430), the Knowledge Innovation Programme of the Chinese Academy of Sciences (KJCX3.SYW.N3), International Atomic Energy Agency (CPR-15818), and the EU sixth Framework Programme (FOOD-CT-2006-016253).References
- P. O. Amoako, P. C. Uden and J. F. Tyson, Anal. Chim. Acta, 2009, 652, 315–323 CrossRef CAS.
- K. Yoshizawa, E. B. Rimm, J. S. Morris, V. L. Spate, C. Hsieh, D. Spiegelman, M. J. Stampfer and W. C. Willett, N. Engl. J. Med., 2002, 347, 1755–1760 CrossRef CAS.
- C. Chen, L. Qu, B. Li, L. Xing, G. Jia, T. Wang, Y. Gao, P. Zhang, M. Li, W. Chen and Z. Chai, Clin. Chem., 2005, 51, 759–767 CrossRef CAS.
- C. Chen, H. Yu, J. Zhao, B. Li, L. Qu, S. Liu, P. Zhang and Z. Chai, Environ. Health Perspect., 2006, 114, 297–301 CrossRef CAS.
- Y.-F. Li, C. Chen, L. Xing, T. Liu, Y. Xie, Y. Gao, B. Li, L. Qu and Z. Chai, Nucl. Technol., 2004, 27, 899–903 CAS.
- K. Seppanen, M. Kantola, R. Laatikainen, K. Nyyssonen, V. P. Valkonen, V. Kaarlopp and J. T. Salonen, J. Trace Elem. Med. Biol., 2000, 14, 84–87 CrossRef CAS.
- Y.-F. Li, C. Chen, B. Li, Q. Wang, J. Wang, Y. Gao, Y. Zhao and Z. Chai, J. Anal. At. Spectrom., 2007, 22, 925–930 RSC.
- L. Kosta, A. R. Byrne and V. Zelenko, Nature, 1975, 254, 238–239 CAS.
- L. H. Reyes, J. M. Marchante-Gayon, J. I. G. Alonso and A. Sanz-Medel, J. Anal. At. Spectrom., 2003, 18, 1210–1216 RSC.
- M. Xu, L. Yang and Q. Wang, J. Anal. At. Spectrom., 2008, 23, 1545–1549 RSC.
- O. Palacios, J. R. Encinar, D. Schaumloffel and R. Lobinski, Anal. Bioanal. Chem., 2006, 384, 1276–1283 CrossRef CAS.
- P. Jitaru, M. Prete, G. Cozzi, C. Turetta, W. Cairns, R. Seraglia, P. Traldi, P. Cescon and C. Barbante, J. Anal. At. Spectrom., 2008, 23, 402–406 RSC.
- P. Jitaru, M. Roman, G. Cozzi, P. Fisicaro, P. Cescon and C. Barbante, Microchim. Acta, 2009, 166, 319–327 CrossRef CAS.
- H. Yu, C. Chen, Y. Gao, B. Li and Z. Chai, Chin. J. Anal. Chem., 2006, 34, 749–753 CAS.
- M. Kotrebai, J. F. Tyson, E. Block and P. C. Uden, J. Chromatogr., A, 2000, 866, 51–63 CrossRef CAS.
- M. Montes-Bayón, T. D. Grant, J. Meija and J. A. Caruso, J. Anal. At. Spectrom., 2002, 17, 1015–1023 RSC.
- S. Afton, K. Kubachka, B. Catron and J. A. Caruso, J. Chromatogr., A, 2008, 1208, 156–163 CrossRef CAS.
- P. Cuderman, I. Kreft, M. Germ, M. Kovacevic and V. Stibilj, J. Agric. Food Chem., 2008, 56, 9114–9120 CrossRef CAS.
- W. Wang, Z. Chen, D. E. Davey and R. Naidu, Microchim. Acta, 2009, 165, 167–172 CrossRef CAS.
- P. Rodrıguez-Gonzalez, J. M. Marchante-Gayon, J. I. G. Alonso and A. Sanz-Medel, Spectrochim. Acta, Part B, 2005, 60, 151–207 CrossRef.
- L. Hu, Z. Dong, X. Huang, Y.-F. Li, B. Li, L. Qu, G. Wang, Y. Gao and C. Chen, Chin. J. Anal. Chem, 2010 Search PubMed , in press.
- J. G. Alonso, Anal. Chim. Acta, 1995, 312, 57–78 CrossRef.
- C. S. Muniz, J. M. M. Gayon, J. I. G. Alonso and A. Sanz-Medel, J. Anal. At. Spectrom., 1999, 14, 1505–1510 RSC.
- G. Alfthan, A. Aro, H. Arvilommi and J. K. Huttunen, Am. J. Clin. Nutr., 1991, 53, 120–125 CAS.
- K. T. Suzuki, C. Sasakura and S. Yoneda, Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol., 1998, 1429, 102–112 Search PubMed.
Footnote |
| † This article is part of a themed issue highlighting outstanding and emerging work in the area of speciation. |
| This journal is © The Royal Society of Chemistry 2011 |
