Magnetically induced colloidal assembly into field-responsive photonic structures
Jianping
Ge
*ab,
Le
He
b,
Yongxing
Hu
b and
Yadong
Yin
*b
aDepartment of Chemistry, Tongji University, Shanghai, China 200092. E-mail: gejianping09@tongji.edu.cn
bDepartment of Chemistry, University of California, Riverside, California 92521, USA. E-mail: yadong.yin@ucr.edu
First published on 28th September 2010
Abstract
Magnetic field is an effective stimulus to assemble magnetic colloidal particles into ordered structures that can display field-responsive photonic properties. Magnetic assembly thus represents a powerful method to produce novel photonic materials with wide applications ranging from various types of color displays to chemical and biological sensing devices. In this article, we review several recent examples of magnetically induced assembly of colloidal particles into photonic structures that show responsive visible-light diffractions. For each case, we discuss the mechanism of assembly and the control of the photonic properties, as well as the advantages and limitations for potential applications.
 Jianping Ge | Jianping Ge received his PhD in Inorganic Chemistry from Tsinghua University in China in 2006, and then spent three years at University of California, Riverside to carry out his postdoctoral research. In 2009, he joined the Department of Chemistry at Tongji University as a professor. His research interests include the synthesis and functionalization of magnetic nanostructures. |
 Le He | Le He received his BS in Chemistry from Nanjing University in 2008. He is now a PhD candidate under the supervision of Prof. Yadong Yin at University of California, Riverside. His research interests include synthesis, self-assembly and functionlization of nanostructured materials. |
 Yongxing Hu | Yongxing Hu received her BS in Material Science and Engineering from University of Science and Technology of China in 2006. She is now a PhD candidate under the supervision of Prof. Yadong Yin at University of California, Riverside. Her research interest includes the self-assembly processes, functionlization of nanostructured materials, and colloidal and interface chemistry. |
 Yadong Yin | Yadong Yin received his BS and MS in Chemistry from the University of Science and Technology of China in 1996 and 1998, respectively, and then PhD in Materials Science and Engineering from the University of Washington in 2002. He then worked as a postdoctoral fellow at the University of California, Berkeley and the Lawrence Berkeley National Laboratory. In 2006 he joined the Department of Chemistry at University of California, Riverside as an assistant professor. His research interests include the synthesis of nanostructured materials and their applications, self-assembly, surface functionalization, and colloidal chemistry. |
1. Introduction
Magnetic assembly of colloidal particles is driven by magnetic dipole–dipole interactions.1 Magnetic dipoles can be approximately considered as tiny bar magnets with opposite poles. The dipole–dipole interaction is directional in nature, and can be either attractive or repulsive, depending on the angle between the magnetic field and the line connecting the dipoles.2,3 Among many types of magnetic materials,4 superparamagnetic colloidal particles are the most suitable building blocks for reversible assembly and manipulation using external magnetic fields. Compared with typical paramagnetic materials, superparamagnetic particles have much higher magnetic susceptibility (usually several orders of magnitude), rendering them significantly more responsive to an external magnetic field. Additionally, their magnetic moments and thereby particle interactions can be fully controlled by the external magnetic field: in the presence of a field the superparamagnetic nanoparticles tend to align along the field due to dipole–dipole interaction, while upon removal of the field, no strong dipole–dipole interaction remains so the particle chains disassemble.5,6Typically, magnetic particles agglomerate to form chain,7–12 string,13 and ring14–16 structures in dilute solutions. When placed in an external magnetic field for an adequately long time, these magnetic particles can form three-dimensional (3D) aggregations as driven by the magnetic packing force towards regions with the maximum magnetic field gradient.17 With fine adjustment of size, magnetic response and surface property of the nanoparticles, various types of force balance can be established which lead to the formation of 1D, 2D, or 3D ordered structures with field-responsive visible-light diffractions.
Compared with conventional colloidal assembly methods such as sedimentation, magnetically induced assembly has several advantages. First, strong magnetic interactions can be induced efficiently and reversibly. Upon the application of an external field, magnetic particles may assemble in one second. Unlike the gravitational field, a magnetic field is tunable with various spatial distributions, created either by permanent magnets or electromagnets, making it capable of assembling particles into complex structures. More importantly, a magnetic field provides a convenient way of controlling the photonic properties of the ordered structures by changing their symmetries or lattice parameters. Taking magnetically responsive photonic crystals as an example, their diffraction wavelength (or band gap position) can be described by Bragg's law, mλ = 2ndsin θ, where m is the diffraction order, λ is the wavelength of incident light, n is the effective refractive index, d is the lattice spacing, and θ is the angle between the incident light and diffraction crystal plane.18–25 The interplanar spacing (d) can be tuned by the magnetic field strength as the induced dipole–dipole interaction is distance-dependent. Magnetic fields may also affect the photonic property by changing the diffraction angle (θ) of the ordered structure because the magnetic dipoles always tend to align parallel to the external field.
The objective of this article is to provide a short review of magnetic field induced self-assembly of colloidal particles and their field-responsive optical diffractions. We start with a brief introduction of magnetic interactions exerted on the colloidal particles, and then discuss a few examples of 1D, 2D and 3D ordered structures with a focus on those systems that have magnetically tunable optical properties. Accompanied by some recent advancements, we try to answer these questions: (i) what is the mechanism for magnetic assembly? (ii) How can one tune the diffraction with a magnetic field? (iii) What are the advantages or limitations for each system? We hope that this review can serve as an easily accessible reference for future research in this field.
2. Magnetic interactions
The magnetization of paramagnetic particles under an external magnetic field follows Langevin function M(x) = Nµ(cothx − (1/x)), where x = µH/(kBT), here N is the number of grains, H the applied field, kB Boltzmann's constant, and T the absolute temperature. The initial slope of the M(H) function is the magnetic susceptibility of the nanoparticleχ. Under low magnetic fields, the induced dipole moment in a superparamagnetic particle can thereby be described as µ = χVHV, where χV is the volume susceptibility of the particle, and V is the volume of the particle. When the external magnetic field is adequately strong, the magnetic moment of the colloidal particle will reach a saturated value.There are two types of magnetic forces for particles with magnetic dipole moments under an external magnetic field. One is the dipole–dipole interactions between particles and the other is the dipole–field force resulting from the magnetic field gradient. As illustrated schematically in Fig. 1, the dipole–dipole interactions can be described as F = 3µ2(1 − 3cos2α)/l4, where µ is the induced magnetic moment, α is the angle between the dipole and the line connecting the dipoles, and l is the center–center distance between two particles. When two dipoles are aligned head-to-end, the dipole interaction is an attraction Fma = −6(µ2/l4). While they are aligned side by side, the interaction becomes a net repulsion Fmr = 3(µ2/l4). As is well known, Brownian motion causes constant movement of colloidal particles in suspension. When the dipole interaction energy Ed = µ2(1 − 3cos2α)/l3 is at least one order of magnitude greater than thermal energy kBT, where kB is Boltzmann's constant and T is absolute temperature, thermal fluctuations can be overcome and self-assembly of particles into chain-like structures can be induced by the external magnetic field. Along the magnetic field, the particles attract each other and form chains due to head-to-end alignment of dipoles while the repulsive interaction, resulting from the side-by-side configuration of dipoles, keeps the chains apart.2,3
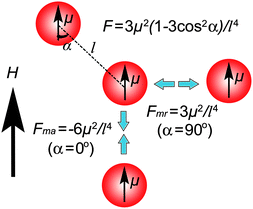 | ||
| Fig. 1 Schematic illustration of the dipole–dipole interactions between two particles with identical magnetic moments in an external magnetic field. An attractive force exists when the dipoles are arranged along the direction of the field, while a repulsive force appears when they are positioned with the connection line perpendicular to the field. | ||
The potential energy of a dipole under an external magnetic field, Zeeman energy, can be expressed as Ep = −µH. The gradient of the magnetic field thus induces a dipole–field force, the packing force, to the particles Fp = ∇(µH), which drives magnetic particles towards regions with the maximum magnetic field strength to minimize their Zeeman energy. The packing force can induce phase separation of colloids from solution and assemble them into 3D structures if the magnetic field is applied for an adequately long time. In many cases, the packing force also plays an important role in both the self-assembly of particles and tuning of optical diffractions.
3. Magnetic assembly of colloids into 1D chains
The 1D chain-like structure is the direct result of dipole–dipole interactions between magnetic particles. When the particles are uniform in size, the interparticle spacing becomes equivalent for all neighboring pairs, thus leading to periodicity along the chain. Since the 1D chain structure is the easiest ordered state switching from the amorphous state, and the steric hindrance for lattice change is negligible in the system, such an ordered structure has inherent advantages in displaying rapid and switchable optical properties.Bibette et al. first discovered that uniform emulsion droplets containing concentrated ferrofluids can instantly organize into ordered structures in the presence of a magnetic field.26,27 The emulsion droplets, composed of a γ-Fe2O3 ferrofluid, were dispersed in water with the help of the ionic surfactant sodium dodecyl sulfate (SDS), and made uniform through a size selection process. In the absence of a magnetic field, the emulsion droplets randomly disperse in water. Once a magnetic field (∼500 Oe) is applied, the monodisperse emulsion droplets form chain structures with a periodic interspacing, which diffract visible light. The diffractions are tuned by the balance of magnetic dipole–dipole attraction and electrostatic repulsion. However, since the emulsion is a thermodynamically unstable system, the long-term stability of the oil droplets against dissociation or aggregation is a concern for practical applications. It was suggested that stability could be enhanced by replacing the emulsion droplets with solid colloidal spheres by polymerization,27 which has been realized in later related works.
Recently, we reported the formation of tunable photonic structures by self-assembling superparamagnetic Fe3O4 colloidal nanocrystal clusters (CNCs) with overall diameters in the range of 100–200 nm, which strongly diffract visible light as an external magnetic field of 100–400 Oe is applied, as shown in Fig. 2a.28–31 The chaining of Fe3O4 CNCs with a periodic interparticle spacing, which is responsible for the optical diffractions, has been verified by both in situ observation using optical microscopy (Fig. 2b)29 and cross-sectional examination using scanning electron microscopy after fixing the assemblies in a polymer matrix (Fig. 2c).32 Further investigation revealed that the establishment of a balance between attraction (which is magnetically induced) and repulsion forces is the key to tuning the interparticle spacing and thereby reversibly controlling the photonic response.
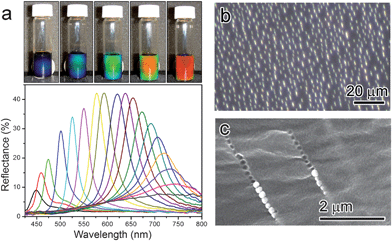 | ||
| Fig. 2 (a) Photographs of colloidal photonic crystals formed in response to an increasing external magnetic field (from right to left), and dependence of reflection spectra on the sample–magnet distance. Diffraction peaks blue-shift as the distance decreases from 3.7 to 2.0 cm with a step size of 0.1 cm. (b) Optical microscopy image of magnetite CNCs assembled under external magnetic field. (c) SEM image of Fe3O4@SiO2 particle chains fixed in a polymer matrix. | ||
The superparamagnetic colloidal particles, synthesized by a high-temperature polyol process, are characterized as superparamagnetic clusters comprising many primary magnetite nanocrystals.33 As they consist almost entirely of magnetite, the CNCs experience strong magnetic attraction which is controllable by changing the strength of the external field. Furthermore, the Fe3O4 CNCs are grafted with a layer of polyelectrolyte (polyacrylate) which provides the surface with high density negative charges in aqueous solution. When the particles are brought together by the magnetic attraction, the electrostatic repulsion counteracts to reach a force balance, leading to the periodic arrangement of particles and consequently Bragg diffraction with a corresponding wavelength. If the force balance is changed, for example, by tuning the strength of the magnetic field, the diffraction wavelength changes accordingly. The resulting photonic structure displays a fast and reversible optical response to the external field owing to the high magnetic content and superparamagnetic characteristics of the building blocks. The responsive frequency can reach ∼30 Hz, but the diffraction intensity gradually drops as only shorter chains can form at higher frequencies.
The electrostatic repulsion is reduced considerably in polar organic systems such as alcohols, so successful assembly is achieved with the assistance of the solvation force at small interparticle distances.30 We have also recently demonstrated that in nonpolar organic systems, the ordered structure can still be established by creating a strong electrostatic repulsion between particles through the charge separation enhancement process using reverse micelles.31 It is now safe to conclude that magnetic assembly of superparamagnetic colloids is a general approach to form dynamically tunable photonic structures if the proper force balance can be established.
4. Magnetic assembly of ferrofluids into 2D hexagonal columns
The formation of 1D chain structures is a general behavior for magnetic particles with sizes ranging from a few nanometres to sub-micrometres. The assembly of magnetic nanoparticles is incapable of diffracting visible light because of the small interparticle spacing. Interestingly, it has been found that ferrofluids composed of superparamagnetic nanocrystals can form 2D ordered structures which also allows the modulation of light propagation in a way similar to an optical grating.34–37Horng et al. reported that magnetic nanoparticles randomly dispersed in a liquid film agglomerated into disordered columns, and then ordered hexagonal columns as the strength of an external “perpendicular” magnetic field increased (Fig. 3a).35 Similar to an optical grating, these particle columns interfere with visible light. The interfered waves will be in phase and have a maximum of diffraction when the path difference between the light meets diffraction equation dsin θ = mλ, where d is the distance between particle columns, θ the angle between the viewing angle and direction norm to the magnetic film, m an integer, and λ the wavelength of diffracted light. Accordingly, the diffracting color is monochromatic only at a fixed angle θ. Due to the misorientation of hexagonal lattices, chromatic rings appear as light passes through the film instead of six diffraction spots that would appear in the case of a perfect hexagonal structure. In fact, observed over a larger range, the diffraction pattern is a set of co-axial rainbow rings with blue inside and red outside, as shown in Fig. 3b.35 The phenomenon can be well explained by the grating equation, since a longer λ leads to a larger θ and also a wider circular ring for a fixed d.
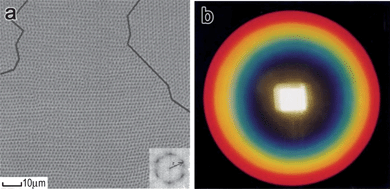 | ||
| Fig. 3 (a) Top view of hexagonal structure of particle columns in a magnetic fluid. (b) Chromatic rings resulting from the diffraction and interference as the beam of white light passing through the magnetic fluid film subjected to a perpendicular magnetic field. Reproduced with permission from ref. 35. | ||
The lattice spacing as well as the colors can be tuned by adjusting parameters such as field strength, film thickness, field sweep rate, and volume fraction of nanoparticles.34,36 The volume fraction of magnetic particles is around 20%, which is much higher than that in a 1D chain system (∼0.1%) and relatively lower than that in a 3D close-packing system (74%). The thickness of the film is designed to be 2–6 µm, as the lattice spacing is roughly proportional to the thickness.38 Generally, a magnetic field with strength of 70–400 Oe is adequate to induce color changing throughout the entire visible spectrum. A stronger field causes the diffraction to blue-shift, probably because more columns form and the average spacing between columns must decrease in a volume-defined dispersion.
Unlike the 1D chain system, such characteristics are unfavorable for producing large area monochromatic images with a macroscale magnetic field. The equilibrium state of the 2D hexagonal columns may be reached within 15 seconds,36 which limits the system to display applications that require no frequent color changes, such as billboards.
5. Magnetic assembly and tuning of 3D colloidal crystals
When a concentrated dispersion of magnetic colloids is exposed to an external field, or a diluted dispersion is placed in a strong field for an adequately long time, the magnetic packing force, together with other interactions and geometry factors, will eventually induce the crystallization of magnetic particles into 3D non-close-packing structures.Sacanna and Philipse reported that a magnetic field induced self-assembly of magnetic poly(methyl methacrylate) latex spheres, which were synthesized by a standard emulsion polymerization method using magnetite-stabilized emulsions as seeds.17 Compared with the gravity field used in most self-assembly techniques, the magnetic field is much more versatile and time-saving, since the strength and spatial distribution of the magnetic field can be well controlled. Similar to the building blocks in conventional assembly, the magnetic colloids must have a narrow size-distribution and a suitable size to Bragg diffract the visible light with a reasonably high intensity. The assembly efficiency may be further improved if the loading of magnetic content (1.7 wt% in this work) can be increased during the synthesis of the composite particles.
Magnetic field can be utilized in combination with gravitational force and capillary force to prepare 3D colloidal crystals in a ‘convective assembly’ process. Song et al. deposited ellipsoidal maghemite particles onto a glass substrate by slowly and vertically pulling the glass out of a colloidal dispersion.39 A magnetic field with the optimal field strength of about 500 Oe was applied to assemble the colloids with a good order in an acceptable process time. While the positional order is achieved by convection, the magnetic field enables the orientational order, which is needed for colloidal particles with symmetry lower than spherical.
In the absence of an external magnetic field, monodispersed superparamagnetic colloidal particles can be assembled using conventional methods because they do not experience strong magnetic dipole–dipole interactions. If the resulting 3D colloidal crystals are not close-packed, magnetic field can serve as a stimulus to induce dipole–dipole interaction between neighboring particles, thus changing the symmetry and lattice parameters of the original colloidal assemblies and enabling tunable photonic properties. As shown in Fig. 4, Asher et al. have developed such a magnetically tunable photonic crystal through the self-assembly of monodisperse highly charged polystyrene colloids which contain ∼17 wt% of superparamagnetic iron oxide nanoparticles.40,41 The colloidal crystal was pre-assembled in deionized water by taking advantage of the strong electrostatic interparticle repulsion, in the form of an fcc lattice with small liquid separations. In an increasing magnetic field gradient from 1.5 to 5.4 kOe cm−1, a blue-shift of diffraction from 560 to 428 nm was observed which can be explained by the compression of colloidal crystal arrays along the magnetic field gradient (Fig. 4b). The tunability of the photonic property is believed to benefit from the higher magnetic loading and surface charge density than those in Philipse's work. Compared with the 1D chain-like structures, the relatively small interparticle separation in this 3D crystal limits the magnitude of change that external fields can induce. It is worth noting that such non-close-packed colloidal crystals can also be fabricated on the surface of an agarose-gel which still allows magnetic tuning of the diffraction property.42
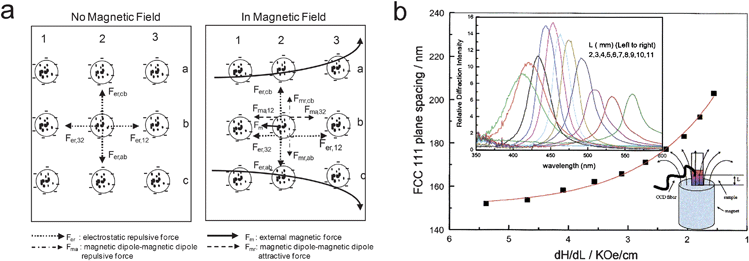 | ||
| Fig. 4 (a) Forces on superparamagnetic particles within a colloidal crystal array in the absence and presence of a magnetic field. (b) Influence of magnetic field gradient, dH/dL, on the lattice constant of a thick CCA composed of 134 nm particles in deionized water (4.2 vol%). Reproduced with permission from ref. 41. | ||
6. Magnetic assembly in confined space
In the above discussions, the magnetic particles are all assembled on a macroscopic scale so that the influence of boundary conditions on the self-assembly is mostly negligible. When carried out in a confined space, however, the colloidal assembly will change its behavior to conform to the boundary conditions. One interesting example is the magnetic assembly of surface modified Fe3O4 CNCs into chain-like structures inside emulsion droplets that are typically tens of micrometres in diameter.43 Upon application of a magnetic field, most particle chains align parallel to the external field as straight strings with periodical lattice spacing, therefore showing monochromatic color. However, due to the combined effects of the magnetically induced strong repulsion between the chains and the spherical confinement of the emulsion droplets, the particle chains near the interface bend to adapt to the curvature of the boundary of the droplets. As a result, the diffraction from the “bent” assemblies blue-shifts since the chains are tilted from the vertical direction with the angle determined by the curvature of the microsphere. As the droplet size becomes smaller, the ratio of surface chains to embedded ones increases so that the overall diffraction of the droplets shows more apparent blue-shifting.We have been able to fix the assembled structures in these micro-droplets by using a UV curable resin as the dispersion medium for modified Fe3O4 CNCs in the emulsion droplets. After magnetic assembly followed by an immediate UV polymerization process, the droplets solidify into microspheres with the ordered structures of CNCs fixed inside. In the SEM images shown in Fig. 5, one can clearly observe the bent chains on the surface of the microspheres. The embedded superparamagnetic chains make the microspheres magnetically anisotropic because the chains assembled under magnetic field always tend to align along the external field direction, which is similar to other magnetically anisotropic structures.44–46 As a result, it is very convenient to rotate the photonic microspheres and change the diffractive colors by controlling the direction of the external field (Fig. 6). The diffraction can be quickly turned “on” and “off” by rotating the external field parallel or perpendicular to the viewing direction. Such photonic microspheres have an additional advantage in that more flavors of color can be realized by simply mixing microspheres of primary colors such as red, green, and blue.
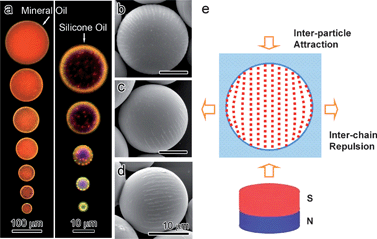 | ||
| Fig. 5 (a) Dark-field optical microscopy images of a series of Fe3O4@SiO2/PEGDA microspheres with diameters from ∼150 to 4 µm. The larger microspheres were fabricated in mineral oil and smaller ones in silicon oil. (b–d) Top view to side view SEM images of the microspheres, showing some of the Fe3O4@SiO2 particle chains aligned on the surface along the longitudinal direction. (e) The scheme illustrating the “bending” of the assembly caused by confined spherical space and strong inter-chain repulsion. Reproduced with permission from ref. 43. | ||
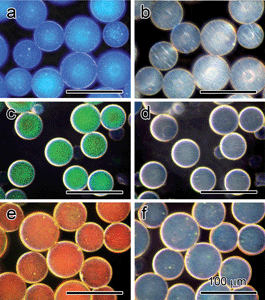 | ||
| Fig. 6 Optical microscopy images of magnetochromatic microspheres with diffractions switched between “on” (a, c and e) and “off” (b, d and f) states by using vertical and horizontal magnetic fields. Reproduced with permission from ref. 43. | ||
7. Conclusions and outlook
We have summarized the recent advancements in the self-assembly of magnetic particles into photonic structures with tunable optical diffractions using external magnetic fields. This is an exciting topic across the fields of both self-assembly and smart responsive photonic materials, which will continue to develop and help to improve the understanding of the magnetic interactions of colloidal particles. At the same time, it allows the design and fabrication of optical components in various photonic devices that require magnetic modulation of electromagnetic waves. More fundamental studies are certainly needed to further simplify the assembly processes, improve the quality and efficiency of magnetic assembly, optimize the tuning range and response speed of the photonic structures, diversify the magnetically responsive systems, and develop methods for incorporating them into practical devices. This article provides a brief introduction to several self-assembly approaches to magnetically tunable photonic structures, and we hope it can stimulate those who are interested in utilizing these field-responsive materials for applications in fields such as optical switches, magnetic sensors, and displays.Acknowledgements
We thank the US National Science Foundation, Department of Energy, and the Donors of the Petroleum Research Fund administered by the American Chemical Society, for support of this research. Yin also thanks the Research Corporation for Science Advancement for the Cottrell Scholar Award and 3M Company for the Nontenured Faculty Grant. Ge thanks Shanghai Pujiang Program (10PJ1409800), National Science Foundation of China (NSFC 21001083), and Tongji University for funding support.References
- K. J. M. Bishop, C. E. Wilmer, S. Soh and B. A. Grzybowski, Small, 2009, 5, 1600 CrossRef CAS.
- R. W. Chabay and B. A. Sherwood, Electric and Magnetic Interactions, Wiley, New York, 1995 Search PubMed.
- Y. Kraftmakher, Eur. J. Phys., 2007, 28, 409 CrossRef.
- U. Jeong, X. W. Teng, Y. Wang, H. Yang and Y. N. Xia, Adv. Mater., 2007, 19, 33 CrossRef CAS.
- S. H. Sun, Adv. Mater., 2006, 18, 393 CrossRef CAS.
- A. F. Rebolledo, A. B. Fuertes, T. Gonzalez-Carreno, M. Sevilla, T. Valdes-Solis and P. Tartaj, Small, 2008, 4, 254 CrossRef CAS.
- K. Butter, P. H. H. Bomans, P. M. Frederik, G. J. Vroege and A. P. Philipse, Nat. Mater., 2003, 2, 88 CrossRef CAS.
- J. H. Gao, B. Zhang, X. X. Zhang and B. Xu, Angew. Chem., Int. Ed., 2006, 45, 1220 CrossRef CAS.
- K. Nakata, Y. Hu, O. Uzun, O. Bakr and F. Stellacci, Adv. Mater., 2008, 20, 4294 CrossRef CAS.
- Z. H. Zhou, G. J. Liu and D. H. Han, ACS Nano, 2009, 3, 165 CrossRef CAS.
- Y. Zhang, L. Sun, Y. Fu, Z. C. Huang, X. J. Bai, Y. Zhai, J. Du and H. R. Zhai, J. Phys. Chem. C, 2009, 113, 8152 CrossRef CAS.
- H. Wang, Q. W. Chen, L. X. Sun, H. P. Qi, X. Yang, S. Zhou and J. Xiong, Langmuir, 2009, 25, 7135 CrossRef CAS.
- J. F. Sun, Y. Zhang, Z. P. Chen, H. Zhou and N. Gu, Angew. Chem., Int. Ed., 2007, 46, 4767 CrossRef CAS.
- S. L. Tripp, S. V. Pusztay, A. E. Ribbe and A. Wei, J. Am. Chem. Soc., 2002, 124, 7914 CrossRef CAS.
- S. L. Tripp, R. E. Dunin-Borkowski and A. Wei, Angew. Chem., Int. Ed., 2003, 42, 5591 CrossRef CAS.
- R. M. Erb, H. S. Son, B. Samanta, V. M. Rotello and B. B. Yellen, Nature, 2009, 457, 999 CrossRef CAS.
- S. Sacanna and A. P. Philipse, Langmuir, 2006, 22, 10209 CrossRef CAS.
- J. M. Weissman, H. B. Sunkara, A. S. Tse and S. A. Asher, Science, 1996, 274, 959 CrossRef CAS.
- S. H. Foulger, P. Jiang, A. Lattam, D. W. Smith, J. Ballato, D. E. Dausch, S. Grego and B. R. Stoner, Adv. Mater., 2003, 15, 685 CrossRef CAS.
- Z. Z. Gu, S. Hayami, Q. B. Meng, T. Iyoda, A. Fujishima and O. Sato, J. Am. Chem. Soc., 2000, 122, 10730 CrossRef CAS.
- M. Ozaki, Y. Shimoda, M. Kasano and K. Yoshino, Adv. Mater., 2002, 14, 514 CrossRef CAS.
- A. C. Arsenault, D. P. Puzzo, I. Manners and G. A. Ozin, Nat. Photonics, 2007, 1, 468 CrossRef CAS.
- J. H. Holtz and S. A. Asher, Nature, 1997, 389, 829 CrossRef CAS.
- S. Y. Choi, M. Mamak, G. von Freymann, N. Chopra and G. A. Ozin, Nano Lett., 2006, 6, 2456 CrossRef CAS.
- H. Fudouzi and Y. N. Xia, Langmuir, 2003, 19, 9653 CrossRef CAS.
- J. Bibette, J. Magn. Magn. Mater., 1993, 122, 37 CrossRef CAS.
- F. Leal Calderon, T. Stora, O. Mondain Monval, P. Poulin and J. Bibette, Phys. Rev. Lett., 1994, 72, 2959 CrossRef CAS.
- J. Ge, Y. Hu and Y. Yin, Angew. Chem., Int. Ed., 2007, 46, 7428 CrossRef CAS.
- J. Ge and Y. Yin, J. Mater. Chem., 2008, 18, 5041 RSC.
- J. Ge and Y. Yin, Adv. Mater., 2008, 20, 3485 CrossRef CAS.
- J. Ge, L. He, J. Goebl and Y. Yin, J. Am. Chem. Soc., 2009, 131, 3484 CrossRef CAS.
- J. Ge, J. Goebl, L. He, Z. Lu and Y. Yin, Adv. Mater., 2009, 21, 4259 CrossRef CAS.
- J. Ge, Y. Hu, M. Biasini, W. P. Beyermann and Y. Yin, Angew. Chem., Int. Ed., 2007, 46, 4342 CrossRef CAS.
- H. E. Horng, C. Y. Hong, W. B. Yeung and H. C. Yang, Appl. Opt., 1998, 37, 2674 CrossRef CAS.
- H. E. Horng, C. Y. Hong, S. L. Lee, C. H. Ho, S. Y. Yang and H. C. Yang, J. Appl. Phys., 2000, 88, 5904 CrossRef CAS.
- H. E. Horng, S. Y. Yang, S. L. Lee, C. Y. Hong and H. C. Yang, Appl. Phys. Lett., 2001, 79, 350 CrossRef CAS.
- S. L. Pu, X. F. Chen, L. J. Chen, W. J. Liao, Y. P. Chen and Y. X. Xia, Appl. Phys. Lett., 2005, 87, 021901 CrossRef.
- H. Wang, Y. Zhu, C. Boyd, W. L. Luo, A. Cebers and R. E. Rosensweig, Phys. Rev. Lett., 1994, 72, 1929 CrossRef CAS.
- T. Ding, K. Song, K. Clays and C. H. Tung, Adv. Mater., 2009, 21, 1936 CrossRef CAS.
- X. L. Xu, G. Friedman, K. D. Humfeld, S. A. Majetich and S. A. Asher, Adv. Mater., 2001, 13, 1681 CrossRef CAS.
- X. L. Xu, G. Friedman, K. D. Humfeld, S. A. Majetich and S. A. Asher, Chem. Mater., 2002, 14, 1249 CrossRef CAS.
- C. Zhu, L. S. Chen, H. Xu and Z. Z. Gu, Macromol. Rapid Commun., 2009, 30, 1945 CrossRef CAS.
- J. Ge, H. Lee, L. He, J. Kim, Z. Lu, H. Kim, J. Goebl, S. Kwon and Y. Yin, J. Am. Chem. Soc., 2009, 131, 15687 CrossRef CAS.
- B. Gates and Y. Xia, Adv. Mater., 2001, 13, 1605 CrossRef CAS.
- X. L. Xu, S. A. Majetich and S. A. Asher, J. Am. Chem. Soc., 2002, 124, 13864 CrossRef CAS.
- S. Y. Park, H. Handa and A. Sandhu, J. Appl. Phys., 2009, 105, 07B526 CrossRef.
| This journal is © The Royal Society of Chemistry 2011 |
