Synthesis and assignment of stereochemistry of the antibacterial cyclic peptide xenematide†
Received
25th June 2010
, Accepted 8th September 2010
First published on 15th October 2010
Abstract
The synthesis of the antimicrobial cyclic peptide xenematide was accomplished by Fmoc solid phase peptide synthesis and the key esterification reaction was achieved using a modified Yamaguchi esterification. Comparison of the optical rotation and NMR data of the synthesized diastereomers to that of the natural product confirmed the structure of xenematide to be PA-L-[Thr-L-Trp-D-Trp-β-Ala]. (PA = phenylacetyl).
1. Introduction
Xenematide is a cyclodepsipeptide isolated from the bacteria Xenorhabdus nematophilus in 2008.1Xenematide exhibits potent antibacterial activity against several bacterial strains including Erwinia amylovora, the pathogen which causes fire blight, a contagious disease that causes the death of apple and pear trees.1,2 Fire blight is an ongoing horticultural problem both internationally and locally. The presence of fire blight in New Zealand restricts fruit exportation to foreign markets; thus effective control over the disease is required. Fire blight is currently controlled with copper solutions, the aminoglycoside antibiotic streptomycin or the less effective antibiotic oxytetracycline.3Streptomycin is used at concentrations of 50–200 μM and is by far the most efficient treatment although streptomycin-resistant strains of E. amylovora have been observed.4,5 Development of novel and effective antibacterial agents such as xenematide is therefore crucial in order to prevent outbreaks of this disease in local horticultural industries. Xenematide has a molecular weight of 662.3 g mol−1 and was shown to consist of one β-alanine (β-Ala) residue, two tryptophan (Trp) residues, one threonine (Thr) residue and one phenylacetyl (PA) group. The threonine residue was determined to be of the L-configuration, whereas both L-and D-tryptophan residues were present in the structure. The order of the L-and D-tryptophan residues within the cyclic peptide could not be assigned at the time of isolation thus two diastereomers are possible. Chemical synthesis of the two diastereomers, namely PA-L-[Thr-D-Trp-L-Trp-β-Ala] and PA-L-[Thr-L-Trp-D-Trp-β-Ala] would allow comparison of their optical rotation and NMR spectra to that of the isolated natural product such that the correct relative and absolute stereochemistry can be unequivocally assigned.
Xenematide (1) can be synthesized by late stage macrolactam formation after cleavage from the resin, via intramolecular cyclization, of the N-terminal β-alanine onto the C-terminal tryptophan residue of resin-bound PA-L-Thr-(L,D)-Trp-(D,L)-Trp-β-Ala 2. Peptide 2 is obtained via esterification of the secondary alcohol on threonine of tripeptide 3 with the carboxylic acid of Boc-β-Ala-OH. Tripeptide 3 in turn is prepared using Fmoc solid phase peptide synthesis (SPPS) starting from either L-or D-Trp-2-ClTrt-resin 4 (Scheme 1).6–8 2-Chlorotrityl-resin (2-ClTrt-resin) was chosen in the current synthesis to minimize C-terminal racemization and the formation of alkylated by-products resulting from cations that can be generated from benzyl-based linkers during TFA-mediated cleavage.9
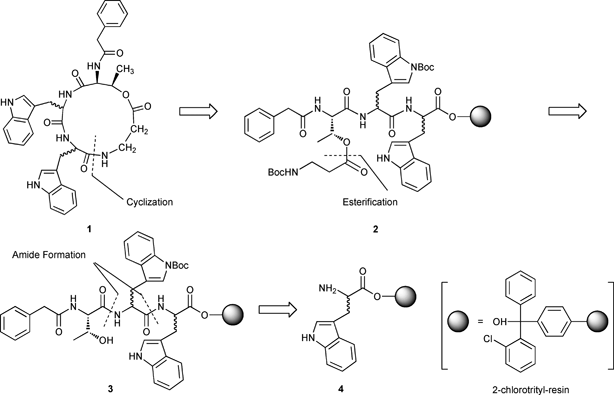 |
| | Scheme 1 Proposed retrosynthesis of xenematide (1). | |
2. Results and discussion
The synthesis of diastereomer PA-L-[Thr-D-Trp-L-Trp-β-Ala] 11 began with amide coupling of Fmoc-D-Trp(Boc)-OH to commercially available H2N-L-Trp-2-ClTrt-resin 5 using HBTU and DIPEA in DMF. After Fmoc deprotection with 20% piperidine solution, dipeptide 6 was coupled to PA-L-Thr-OH prepared according to literature procedure10 affording tripeptide 7. Esterification between the carboxylic acid group on Boc-β-Ala-OH and the hydroxyl group on threonine of tripeptide 7 was then attempted using DIC/DMAP11 but the reaction did not proceed even after extended reaction times under microwave irradiation (Scheme 2).
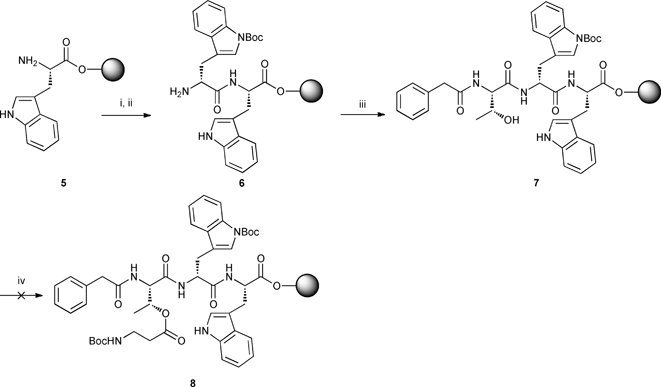 |
| | Scheme 2 Synthesis of tripeptide 7 and attempted esterification of Boc-β-Ala-OH with L-threonine. Reagents, conditions and yields: i) Fmoc-D-Trp(Boc)-OH (3 equiv.), HBTU, DIPEA, DMF, rt, 45 min; ii) 20% piperidine/DMF, rt; iii) PA-L-Thr-OH (3 equiv.), HBTU, DIPEA, DMF, rt, 45 min; iv) Boc-β-Ala-OH (3 equiv.), DIC, DMAP, DMF, Δ, microwave. | |
After extensive investigation of the formation of the ester bond between threonine and Boc-β-Ala-OH, it was found that the use of modified Yamaguchi esterification conditions12 (BzCl, Et3N and catalytic DMAP in THF) afforded the desired dipeptide 9 in satisfactory yield in the solution phase reaction (Scheme 3). Unfortunately, subsequent removal of the benzyl group by hydrogenolysis followed by coupling to dipeptide 6 using HBTU and DIPEA in DMF resulted in the formation of a product with m/z value of 462.2. This undesired product 10, identified as β-Ala-D-Trp-L-Trp, was postulated to form viaβ-Ala transfer from threonine to tryptophan during the peptide coupling process (Scheme 3).
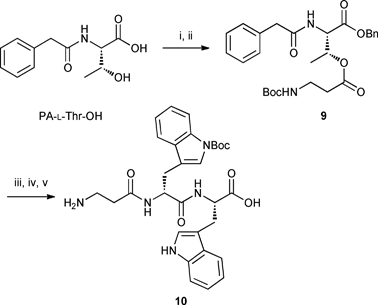 |
| | Scheme 3 Esterification using modified Yamaguchi conditions and formation of undesired product 10. Reagents, conditions and yields: i) Et3N, BnBr, DMF, rt, 16 h, 73%; ii) Boc-β-Ala-OH, BzCl, Et3N, DMAP, THF, rt, 88 h, 71%; iii) H2, 10% Pd/C, MeOH, rt, overnight, 93%; iv) compound 6, HBTU, DIPEA, DMF, rt, 45 min; v) TFA : H2O : TIPS (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5), 1 h. 2.5), 1 h. | |
Esterification using modified Yamaguchi conditions was next performed on resin-bound peptide 7, forming the desired peptide 8 in excellent yield with none of the undesired product 10 being detected (Scheme 4). CH2Cl2 was found to be a superior solvent to DMF and use of excess reagents resulted in a shortened reaction time with quantitative conversion. Following cleavage from the resin using a mixture of TFA/H2O/TIPS (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5), intramolecular cyclization was carried out using BOPCl and DMAP13 to give the diastereomer PA-L-[Thr-D-Trp-L-Trp-β-Ala] 11 (Scheme 4).
2.5), intramolecular cyclization was carried out using BOPCl and DMAP13 to give the diastereomer PA-L-[Thr-D-Trp-L-Trp-β-Ala] 11 (Scheme 4).
![Synthesis of PA-l-[Thr-d-Trp-l-Trp-β-Ala] 11. Reagents, conditions and yields: i) Boc-β-Ala-OH (20 equiv.), BzCl (20 equiv.), Et3N (40 equiv.), CH2Cl2, rt, 18 h; ii) TFA : H2O :TIPS (95 : 2.5 : 2.5), 1 h; iii) BOPCl, DMAP, CH2Cl2–MeOH, 0 °C to rt, 19 h, 8% from compound 5.](/image/article/2011/OB/c0ob00315h/c0ob00315h-s4.gif) |
| | Scheme 4 Synthesis of PA-L-[Thr-D-Trp-L-Trp-β-Ala] 11. Reagents, conditions and yields: i) Boc-β-Ala-OH (20 equiv.), BzCl (20 equiv.), Et3N (40 equiv.), CH2Cl2, rt, 18 h; ii) TFA : H2O :TIPS (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5), 1 h; iii) BOPCl, DMAP, CH2Cl2–MeOH, 0 °C to rt, 19 h, 8% from compound 5. 2.5), 1 h; iii) BOPCl, DMAP, CH2Cl2–MeOH, 0 °C to rt, 19 h, 8% from compound 5. | |
Alternatively, the other possible diastereomer PA-L-[Thr-L-Trp-D-Trp-β-Ala] 12 was synthesized in a similar manner starting from D-Trp(Boc)-2-ClTrt-resin 13 (Scheme 5). Upon comparison of the optical rotation and NMR data (1H and 13C) of the synthesized peptides to that of the natural product (Tables 1–3), it was established that xenematide contains the peptide sequence PA-L-[Thr-L-Trp-D-Trp-β-Ala] 12.
![Synthesis of PA-l-[Thr-l-Trp-d-Trp-β-Ala] 12. Reagents, conditions and yields: i) Fmoc-l-Trp(Boc)-OH (3 equiv.), HBTU, DIPEA, DMF, rt, 45 min; ii) 20% piperidine/DMF, rt; iii) PA-l-Thr-OH (3 equiv.), HBTU, DIPEA, DMF, rt, 45 min; iv) Boc-β-Ala-OH (20 equiv.), BzCl (20 equiv.), Et3N (40 equiv.), CH2Cl2, rt, 18 h; v) TFA : H2O : TIPS (95 : 2.5 : 2.5), 1 h; vi) BOPCl, DMAP, CH2Cl2–MeOH, 0 °C to rt, 19 h, 4% from compound 13.](/image/article/2011/OB/c0ob00315h/c0ob00315h-s5.gif) |
| | Scheme 5 Synthesis of PA-L-[Thr-L-Trp-D-Trp-β-Ala] 12. Reagents, conditions and yields: i) Fmoc-L-Trp(Boc)-OH (3 equiv.), HBTU, DIPEA, DMF, rt, 45 min; ii) 20% piperidine/DMF, rt; iii) PA-L-Thr-OH (3 equiv.), HBTU, DIPEA, DMF, rt, 45 min; iv) Boc-β-Ala-OH (20 equiv.), BzCl (20 equiv.), Et3N (40 equiv.), CH2Cl2, rt, 18 h; v) TFA : H2O : TIPS (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5), 1 h; vi) BOPCl, DMAP, CH2Cl2–MeOH, 0 °C to rt, 19 h, 4% from compound 13. 2.5), 1 h; vi) BOPCl, DMAP, CH2Cl2–MeOH, 0 °C to rt, 19 h, 4% from compound 13. | |
| Position |
δ
C, multiplicity |
δ
C (J in Hz) |
Position |
δ
C, multiplicity |
δ
C (J in Hz) |
|
PA = phenylacetyl. [α]20D +45.0 (c 0.20 in MeOH).
|
|
β-Ala
|
169.2, qC |
|
Trp–4 |
109.3, qC |
|
| |
33.9, CH2 |
2.52, m |
–5 |
127.0, qC |
|
| |
|
2.40, m |
–6 |
118.2, CH |
7.55, d (7.8) |
| |
34.6, CH2 |
3.39, m |
–7 |
118.2, CH |
7.00, td (7.8, 1.0) |
| |
|
3.32, m |
–8 |
121.0, CH |
7.08, m |
|
β-Ala-NH
|
|
7.39, t (6.1) |
–9 |
111.3, CH |
7.36, bd (8.0) |
| Trp–1 |
171.0, qC |
|
–10 |
136.0, qC |
|
| –2 |
54.5, CH |
4.19, ddd (10.0, 7.9, 3.7) |
–11 |
|
10.7, s |
| –3 |
25.7, CH2 |
3.18, m |
–12 |
123.4, CH |
7.09, m |
| |
|
2.87, m |
Trp-NH |
|
8.81, d (6.7) |
| –4 |
110.6, qC |
|
Thr |
170.2, qC |
|
| –5 |
126.9, qC |
|
|
54.0, CH |
4.65, dd (9.2, 2.2) |
| –6 |
117.9, CH |
7.49, d (7.8) |
|
72.0, CH |
5.11, qd (6.3, 2.3) |
| –7 |
118.2, CH |
6.96, m |
|
16.2, CH3 |
1.05, d (6.2) |
| –8 |
120.8, CH |
7.05, td (7.8, 1.0) |
Thr-NH |
|
8.09, d (9.5) |
| –9 |
111.3, CH |
7.33, bd (8.0) |
PAa |
170.6, qC |
|
| –10 |
135.9, qC |
|
|
41.7, CH2 |
3.65, d (14.1) |
| –11 |
|
10.6, s |
|
|
3.55, d (14.1) |
| –12 |
123.1, CH |
6.96, m |
|
136.3, qC |
|
| Trp-NH |
|
8.72, d (6.7) |
|
129.0, CH |
7.26, m |
| Trp–1 |
172.0, qC |
|
|
128.1, CH |
7.29, m |
| –2 |
54.0, CH |
4.53, q (7.3) |
|
126.1, CH |
7.19, m |
| –3 |
25.7, CH2 |
2.82–2.92, m |
|
|
|
Table 2
δ
H(300 MHz; DMSO-d6) and δC(75 MHz; DMSO-d6) of PA-L-[Thr-D-Trp-L-Trp-β-Ala] 11
| Position |
δ
C, multiplicity |
δ
C (J in Hz) |
Position |
δ
C, multiplicity |
δ
C (J in Hz) |
|
PA = phenylacetyl.
δ
H Peaks for PhCH2CON are not included as they are obscured by DMSO-d6. [α]20D −36.9 (c 0.52 in MeOH).
|
|
β-Ala
|
169.70, qC |
|
Trp–4 |
109.98, qC |
|
| |
34.77, CH2 |
2.28–2.44, m |
–5 |
127.59, qC |
|
| |
35.21, CH2 |
3.55–3.59, m |
–6 |
118.77, CH |
7.46, d (7.8) |
|
β-Ala-NH
|
|
7.22–7.35, m |
–7 |
118.77, CH |
6.91–6.95, m |
| Trp–1 |
171.52, qC |
|
–8 |
121.39, CH |
6.91–6.95, m |
| –2 |
55.32, CH |
4.30–4.34, m |
–9 |
111.85, CH |
7.22–7.35, m |
| –3 |
26.45, CH2 |
2.74–2.95, m |
–10 |
136.47, qC |
|
| –4 |
110.93, qC |
|
–11 |
|
10.69, s |
| –5 |
127.59, qC |
|
–12 |
123.66, CH |
6.91–6.95, m |
| –6 |
118.54, CH |
7.41, d (7.8) |
Trp-NH |
|
8.44–8.59, m |
| –7 |
118.54, CH |
6.91–6.95, m |
Thr |
171.30, qC |
|
| –8 |
121.33, CH |
6.91–6.95, m |
|
56.59, CH |
4.44–4.48, m |
| –9 |
111.85, CH |
7.22–7.35, m |
|
70.43, CH |
5.41–5.43, m |
| –10 |
136.47, qC |
|
|
16.95, CH3 |
1.03, d (6.0) |
| –11 |
|
10.69, s |
Thr-NH |
|
7.82, d (7.8) |
| –12 |
123.66, CH |
6.91–6.95, m |
PAa |
171.45, qC |
|
| Trp-NH |
|
8.44–8.59, m |
|
42.56, CH2 |
—b |
| Trp–1 |
171.76, qC |
|
|
136.85, qC |
|
| –2 |
53.70, CH |
4.53–4.57, m |
|
129.48, CH |
7.22–7.35, m |
| –3 |
27.04, CH2 |
3.12–3.17, m |
|
128.75, CH |
7.22–7.35, m |
| |
|
|
|
126.90, CH |
7.22–7.35, m |
Table 3
δ
H(300 MHz; DMSO-d6) and δC(75 MHz; DMSO-d6) of PA-L-[Thr-L-Trp-D-Trp-β-Ala] 12
| Position |
δ
C, multiplicity |
δ
C (J in Hz) |
Position |
δ
C, multiplicity |
δ
C (J in Hz) |
|
PA = phenylacetyl. [α]20D +61.0 (c 0.58 in MeOH).
|
|
β-Ala
|
169.78, qC |
|
Trp–4 |
109.82, qC |
|
| |
34.48, CH2 |
2.37–2.49, m |
–5 |
127.49, qC |
|
| |
|
|
–6 |
118.81, CH |
7.54, d (7.8) |
| |
35.13, CH2 |
3.39–3.41, m |
–7 |
118.81, CH |
6.93–7.09, m |
| |
|
|
–8 |
121.53, CH |
6.93–7.09, m |
|
β-Ala-NH
|
|
7.17–7.42, m |
–9 |
111.90, CH |
7.17–7.42, m |
| Trp–1 |
171.61, qC |
|
–10 |
136.54, qC |
|
| –2 |
55.07, CH |
4.17, ddd (10.2, 6.6, 3.6) |
–11 |
|
10.72, s |
| –3 |
26.25, CH2 |
3.16–3.21, m |
–12 |
123.96, CH |
6.93–7.09, m |
| –4 |
111.17, qC |
|
Trp-NH |
|
8.82, d (6.9) |
| –5 |
127.40, qC |
|
Thr |
170.83, qC |
|
| –6 |
118.49, CH |
7.48, d (7.8) |
|
54.53, CH |
4.62–4.65, m |
| –7 |
118.81, CH |
6.93–7.09, m |
|
72.54, CH |
5.09–5.11, m |
| –8 |
121.38, CH |
6.93–7.09, m |
|
16.70, CH3 |
1.04, d (6.0) |
| –9 |
111.84, CH |
7.17–7.42, m |
Thr-NH |
|
8.13, d (6.9) |
| –10 |
136.54, qC |
|
PAa |
171.20, qC |
|
| –11 |
|
10.63, s |
|
42.17, CH2 |
3.65, d (14.1) |
| –12 |
123.70, CH |
6.93–7.09, m |
|
|
3.54, d (14.1) |
| Trp-NH |
|
8.76, d (6.9) |
|
136.83, qC |
|
| Trp–1 |
172.58, qC |
|
|
129.55, CH |
7.17–7.42, m |
| –2 |
54.44, CH |
4.52, q (7.2) |
|
128.64, CH |
7.17–7.42, m |
| –3 |
26.25, CH2 |
2.82–2.95, m |
|
126.78, CH |
7.17–7.42, m |
In addition to the two possible diastereomers of the natural cyclic peptide xenematide, two more unnatural diastereomers with the peptide sequences, PA-L-[Thr-L-Trp-L-Trp-β-Ala] and PA-L-[Thr-D-Trp-D-Trp-β-Ala], were also synthesized using the same synthetic strategy. It was envisaged that these additional cyclic peptides could be used to probe the bioactivity of xenematide.
3. Conclusions
The synthesis of the antimicrobial cyclic peptide xenematide was accomplished by Fmoc solid phase peptide synthesis with the key esterification reaction being achieved using a modified Yamaguchi esterification. Comparison of the optical rotation and NMR data of the synthesized diastereomers to that of the natural product confirmed the structure of xenematide to be PA-L-[Thr-L-Trp-D-Trp-β-Ala]. (PA = phenylacetyl). The antibacterial activity of the synthetic peptides and the design of further peptidomimetic analogues of xenematide are currently being investigated.
4. Experimental
4.1 Synthesis of (2S,3R)-benzyl 3-[3–(tert-butoxycarbonyl amino)propanoyloxy]-2-(2-phenylacetamido)butanoate 910,12
4.1.1 Synthesis of (2S,3R)-benzyl 2-hydroxy-2-(2-phenyl acetamido)butanoate (PA-L-Thr-OBn).
To a solution of L-threonine (5.42 g, 45.49 mmol) in 1 M aqueous NaOH solution (150 mL) at 0 °C was added phenylacetyl chloride (7.85 mL, 59.12 mmol) dropwise, and the reaction was stirred at 0 °C for 1 h. Phenylacetyl chloride (7.85 mL, 59.12 mmol) was added at 0 °C and the reaction mixture was warmed to room temperature and stirred for 18 h. After extraction with ethyl acetate (90 mL), the aqueous layer was acidified with 3 M HCl solution to pH 2 and was extracted with ethyl acetate (3 × 120 mL). The combined organic extracts were dried over anhydrous Na2SO4, filtered, concentrated in vacuo to give a colourless solid which was washed with cold diethyl ether and used without further purification. To a solution of the above solid (6.03 g, 25.44 mmol) in DMF (28 mL) at room temperature was added triethylamine (4.25 mL, 30.49 mmol), benzyl bromide (6.63 mL, 30.52 mmol), and the reaction mixture was stirred for 16 h. Water (24 mL) and dichloromethane (60 mL) were added and the suspension was stirred for 15 min. The organic layer was separated and the aqueous layer was extracted with dichloromethane (3 × 60 mL). The combined organic extracts were dried over anhydrous Na2SO4, filtered and concentrated in vacuo. Washing with cold diethyl ether afforded the desired product (6.07 g, 73%) as a colourless solid; Rf 0.41 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EtOAc–hexane); mp 150.5–152.8 °C; vmax(film)/cm−1 3469, 3349, 1705, 1648, 1529, 1279, 1001, 725 and 693; [α]20D −3.6 (c 1.11 in CDCl3); δH(300 MHz; CDCl3) 1.04 (3H, d, J 6.0, Thrβ–CH3), 3.55 (2H, s, PhCH2CON), 4.25 (1H, dq, J 3.0 and 6.0, Thrβ–CH), 4.48–4.52 (1H, m, Thrα–CH), 5.07 (2H, s, PhCH2CO2), 6.88 (1H, d, CONHCH), 7.17–7.27 (10H, m, Ph); δC(75 MHz; CDCl3) 19.6 (CH3, Thrβ–CH3), 42.95 (CH2, PhCH2CON), 43.0 (CH2, PhCH2CON), 57.6 (CH, Thrα–CH), 57.65 (CH, Thrα–CH), 67.1 (CH2, PhCH2CO2), 67.2 (CH, Thrβ–CH), 126.9 (CH, Ph), 127.9 (CH, Ph), 128.1 (CH, Ph), 128.2 (CH, Ph), 128.4 (CH, Ph), 128.6 (CH, Ph), 129.0 (CH, Ph), 129.6 (CH, Ph), 132.75 (quat., Ph), 134.4 (quat., Ph), 135.1 (quat., Ph), 170.6 (quat., CHCO2CH2), 172.1 (quat., CH2CON), 172.2 (quat., CH2CON); m/z (EI) 328.1531 (MH+, C19H22NO4 requires 328.1543), 328 (MH+, 6%), 350 (MNa+, 100%), 351 (20) and 352 (2).
1 EtOAc–hexane); mp 150.5–152.8 °C; vmax(film)/cm−1 3469, 3349, 1705, 1648, 1529, 1279, 1001, 725 and 693; [α]20D −3.6 (c 1.11 in CDCl3); δH(300 MHz; CDCl3) 1.04 (3H, d, J 6.0, Thrβ–CH3), 3.55 (2H, s, PhCH2CON), 4.25 (1H, dq, J 3.0 and 6.0, Thrβ–CH), 4.48–4.52 (1H, m, Thrα–CH), 5.07 (2H, s, PhCH2CO2), 6.88 (1H, d, CONHCH), 7.17–7.27 (10H, m, Ph); δC(75 MHz; CDCl3) 19.6 (CH3, Thrβ–CH3), 42.95 (CH2, PhCH2CON), 43.0 (CH2, PhCH2CON), 57.6 (CH, Thrα–CH), 57.65 (CH, Thrα–CH), 67.1 (CH2, PhCH2CO2), 67.2 (CH, Thrβ–CH), 126.9 (CH, Ph), 127.9 (CH, Ph), 128.1 (CH, Ph), 128.2 (CH, Ph), 128.4 (CH, Ph), 128.6 (CH, Ph), 129.0 (CH, Ph), 129.6 (CH, Ph), 132.75 (quat., Ph), 134.4 (quat., Ph), 135.1 (quat., Ph), 170.6 (quat., CHCO2CH2), 172.1 (quat., CH2CON), 172.2 (quat., CH2CON); m/z (EI) 328.1531 (MH+, C19H22NO4 requires 328.1543), 328 (MH+, 6%), 350 (MNa+, 100%), 351 (20) and 352 (2).
4.1.2 Synthesis of (2S,3R)-benzyl 3-[3-(tert-butoxycarbonyl amino)propanoyloxy]-2-(2-phenylacetamido)butanoate 9.
To a solution of PA-L-Thr-OBn (1.70 g, 5.19 mmol), Boc-β-Ala-OH (0.98 g, 5.19 mmol), and benzoyl chloride (0.61 mL, 5.25 mmol) in dry THF (47 mL) at room temperature under N2 was added dropwise triethylamine (1.45 mL, 10.38 mmol) and DMAP (0.16 g, 1.31 mmol), and the reaction mixture was stirred for 88 h. The solvent was removed in vacuo and the residue was purified by flash column chromatography (EtOAc–hexane 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to give compound 9 (1.85 g, 71%) as a yellow oil; Rf 0.39 (1
2) to give compound 9 (1.85 g, 71%) as a yellow oil; Rf 0.39 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 EtOAc–hexane); vmax(film)/cm−1 3302, 1739, 1663, 1519, 1164, 731 and 697; [α]20D +17.3 (c 2.61 in CDCl3); δH(300 MHz; CDCl3) 1.17 (3H, d, J 6.0, Thrβ–CH3), 1.44 [9H, s, C(CH3)3], 2.21–2.30 (2H, m, CO2CH2CH2NH), 3.16–3.27 (2H, m, CO2CH2CH2NH), 3.67 (2H, s, PhCH2CON), 4.79 (1H, d, J 9.0, Thrα–CH), 5.09 (2H, d, PhCH2CO2), 5.38 (1H, d, J 3.0, Thrβ–CH), 7.26–7.37 (10H, m, Ph); δC(75 MHz; CDCl3) 16.9 (CH3, Thrβ–CH3), 28.3 [CH3, C(CH3)3], 34.6 (CH2, β–Ala–CH2), 35.9 (CH2, β–Ala–CH2), 43.3 (CH2, PhCH2CON), 55.5 (CH, Thrα–C), 67.5 (CH2, PhCH2CO2), 70.6 (CH, Thrβ–C), 79.4 [quat., C(CH3)3], 127.3 (CH, Ph), 128.3 (CH, Ph), 128.5 (CH, Ph), 128.6 (CH, Ph), 128.8 (CH, Ph), 129.3 (CH, Ph), 129.9 (CH, Ph), 133.1 (CH, Ph), 134.5 (quat., Ph), 134.9 (quat., Ph), 155.8 (quat., NCO2C), 169.5 (quat., CHCO2CH2, CHCO2CH2), 170.5 (quat., CH2CON); m/z (EI) 521.2256 (MNa+, C27H34N2NaO7 requires 521.2258), 521 (MNa+, 100%), 522 (48), 523 (10), 537 (20) and 538 (4).
2 EtOAc–hexane); vmax(film)/cm−1 3302, 1739, 1663, 1519, 1164, 731 and 697; [α]20D +17.3 (c 2.61 in CDCl3); δH(300 MHz; CDCl3) 1.17 (3H, d, J 6.0, Thrβ–CH3), 1.44 [9H, s, C(CH3)3], 2.21–2.30 (2H, m, CO2CH2CH2NH), 3.16–3.27 (2H, m, CO2CH2CH2NH), 3.67 (2H, s, PhCH2CON), 4.79 (1H, d, J 9.0, Thrα–CH), 5.09 (2H, d, PhCH2CO2), 5.38 (1H, d, J 3.0, Thrβ–CH), 7.26–7.37 (10H, m, Ph); δC(75 MHz; CDCl3) 16.9 (CH3, Thrβ–CH3), 28.3 [CH3, C(CH3)3], 34.6 (CH2, β–Ala–CH2), 35.9 (CH2, β–Ala–CH2), 43.3 (CH2, PhCH2CON), 55.5 (CH, Thrα–C), 67.5 (CH2, PhCH2CO2), 70.6 (CH, Thrβ–C), 79.4 [quat., C(CH3)3], 127.3 (CH, Ph), 128.3 (CH, Ph), 128.5 (CH, Ph), 128.6 (CH, Ph), 128.8 (CH, Ph), 129.3 (CH, Ph), 129.9 (CH, Ph), 133.1 (CH, Ph), 134.5 (quat., Ph), 134.9 (quat., Ph), 155.8 (quat., NCO2C), 169.5 (quat., CHCO2CH2, CHCO2CH2), 170.5 (quat., CH2CON); m/z (EI) 521.2256 (MNa+, C27H34N2NaO7 requires 521.2258), 521 (MNa+, 100%), 522 (48), 523 (10), 537 (20) and 538 (4).
4.2 Synthesis of H2N-D-Trp(Boc)-2-ClTrt-aminomethyl polystyrene resin 136–8
To aminomethyl polystyrene resin (0.1 g) was added a mixture of 2-chloro-4′-carboxytriphenylmethanol (68.8 mg, 0.2 mmol) and N,N-diisopropylcarbodiimide (21.37 μL, 0.2 mmol) in DMF (1 mL), and the reaction was stirred for 1 h. The resin was washed with DMF (2 × 1 mL), dichloromethane (2 × 1 mL), methanol (3 × 1 mL), diethyl ether (2 × 1 mL) and then dried under N2. To the above resin was added dry thionyl chloride/dichloromethane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 4 mL) dropwise, and the reaction was gently stirred at room temperature for 3 h. The resin was washed with DMF (2 × 1 mL), dichloromethane (3 × 1 mL) and then dried for 10 min. To this resin was added a solution of Fmoc-D-Trp(Boc)-OH (0.12 g, 0.2 mmol) and DIPEA (92.1 μL, 0.5 mmol) in dry dichloromethane (2 mL), and the reaction was gently stirred at room temperature for 30 min. After washing with DMF (2 × 1 mL), a solution of CH2Cl2/MeOH/DIPEA (80
1 v/v, 4 mL) dropwise, and the reaction was gently stirred at room temperature for 3 h. The resin was washed with DMF (2 × 1 mL), dichloromethane (3 × 1 mL) and then dried for 10 min. To this resin was added a solution of Fmoc-D-Trp(Boc)-OH (0.12 g, 0.2 mmol) and DIPEA (92.1 μL, 0.5 mmol) in dry dichloromethane (2 mL), and the reaction was gently stirred at room temperature for 30 min. After washing with DMF (2 × 1 mL), a solution of CH2Cl2/MeOH/DIPEA (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v, 10 mL) was added, the reaction was stirred for 10 min and repeated. After washing with DMF (3 × 1 mL), a 20% piperidine/DMF solution (v/v, 10 mL) was added and the reaction was stirred for 3 min and then repeated for 20 min. The resin was washed with DMF (6 × 1 mL), iPrOH (3 × 1 mL), hexane (4 × 1 mL), air dried for 15 min and then dried under N2. The loading of Fmoc-D-Trp(Boc)-OH was found to be 0.39 mmol g−1 (theoretical loading = 0.58 mmol g−1, 67%) using the Fmoc assay.14
5 v/v, 10 mL) was added, the reaction was stirred for 10 min and repeated. After washing with DMF (3 × 1 mL), a 20% piperidine/DMF solution (v/v, 10 mL) was added and the reaction was stirred for 3 min and then repeated for 20 min. The resin was washed with DMF (6 × 1 mL), iPrOH (3 × 1 mL), hexane (4 × 1 mL), air dried for 15 min and then dried under N2. The loading of Fmoc-D-Trp(Boc)-OH was found to be 0.39 mmol g−1 (theoretical loading = 0.58 mmol g−1, 67%) using the Fmoc assay.14
4.3 Synthesis of PA-L-[Thr-D-Trp-L-Trp-β-Ala] 11
4.3.1 Synthesis of PA-L-Thr-D-Trp-L-Trp-2-ClTrt-resin 7.
To H2N-L-Trp-2-ClTrt-resin (0.56 mmol g−1) (0.18 g, 0.1 mmol) was added a mixture of Fmoc-D-Trp(Boc)-OH (0.16 g, 0.31 mmol), HBTU (0.11 g, 0.29 mmol) and DIPEA (0.11 mL, 0.61 mmol) in DMF (1 mL), and the reaction was agitated for 45 min. The resin was washed with DMF (6 × 1 mL), isopropanol (3 × 1 mL), hexane (4 × 1 mL) and then dried under N2. A solution of 20% piperidine/DMF (v/v, 10 mL) was added and the reaction was gently agitated for 5 min. The resin was washed with DMF (6 × 1 mL), and the same procedure was repeated for 10 min. After washing with DMF (6 × 1 mL), a mixture of PA-L-Thr-OH (0.08 g, 0.33 mmol), HBTU (0.11 g, 0.29 mmol) and DIPEA (0.11 mL, 0.61 mmol) in DMF (1 mL) was added, and the reaction was agitated for 45 min. The resin was washed with DMF (6 × 1 mL), isopropanol (3 × 1 mL), hexane (4 × 1 mL) and then dried under N2.
4.3.2 Synthesis of PA-L-Thr(Boc-β-Ala)-D-Trp-L-Trp-2-ClTrt-resin 8.
To peptide 7 was added a mixture of Boc-β-Ala-OH (0.39 g, 2.05 mmol), benzoyl chloride (0.24 mL, 2.04 mmol), triethylamine (0.57 mL, 4.08 mmol) and DMAP (4.7 mg, 0.04 mmol) in dichloromethane (10 mL) and the reaction was agitated for 18 h. The resin was washed with DMF (6 × 1 mL), isopropanol (3 × 1 mL), hexane (4 × 1 mL) and dried under N2.
4.3.3 Synthesis of PA-L-[Thr-D-Trp-L-Trp-β-Ala] 1113.
To peptide 8 was added a mixture of TFA/H2O/TIPS (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 v/v, 10 mL) and the reaction was agitated for 1 h. The solution was filtered and concentrated in vacuo. To the resultant yellow residue (69.5 mg, 0.1 mmol) was dissolved in dichloromethane–methanol (4
2.5 v/v, 10 mL) and the reaction was agitated for 1 h. The solution was filtered and concentrated in vacuo. To the resultant yellow residue (69.5 mg, 0.1 mmol) was dissolved in dichloromethane–methanol (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 139 mL) at 0 °C, BOPCl (0.13 g, 0.51 mmol) and DMAP (0.11 g, 0.92 mmol) were added, and the reaction was stirred for 19 h. 1 M HCl solution (30 mL) was added and the aqueous layer was extracted with dichloromethane (3 × 80 mL). The combined organic extracts were dried over anhydrous Na2SO4, filtered and concentrated in vacuo. Purification by semi-preparative RP–HPLC (using a linear gradient of 40% B to 70% B) yielded the title compound (8% from H2N-L-Trp-2-ClTrt-resin) as an off-white amorphous solid in >99% purity according to analytical RP-HPLC; Rt 10.77 min (XTerra C18, 4.6 × 150 mm, 30% to 75% B over 15 min, 1 mL min−1); mp 150.2–156.4 °C; vmax(film)/cm−1 3315, 1736, 1649, 1514, 1165, 1060, 743 and 697; [α]20D −36.9 (c 0.52 in MeOH); δH(300 MHz; DMSO-d6)* 1.03 (3H, d, Thrβ–CH3), 2.28–2.44 (2H, m, β–Ala–CH2), 2.74–2.95 (3H, m, Trpβ–CH2), 3.12–3.17 (1H, m, Trpβ–CH2), 3.55–3.59 (2H, m, β–Ala–CH2), 4.30–4.34 (1H, m, Trpα–CH), 4.44–4.48 (1H, m, Thrα–CH), 4.53–4.57 (1H, m, Trpα–CH), 5.41–5.43 (1H, m, Thrβ–CH), 6.91–6.95 (6H, m, Trp–H7, Trp–H8, Trp–H12), 7.22–7.35 (8H, m, β–Ala–NH, Trp–H9, PA–Ph), 7.41 (1H, d, J 7.8, Trp–H6), 7.46 (1H, d, J 7.8, Trp–H6), 7.82 (1H, d, J 7.8, Thr–CONH), 8.44–8.59 (2H, m, Trp–CONH), 10.69 (2H, s, Trp–H11); δC(75 MHz, DMSO-d6) 16.95 (CH3, Thrβ–CH3), 26.45 (CH2, Trpβ–CH2), 27.04 (CH2, Trpβ–CH2), 34.77 (CH2, β-Ala–CH2), 35.21 (CH2, β-Ala–CH2), 42.56 (CH2, PhCH2CON), 53.70 (CH, Trpα–CH), 55.32 (CH, Trpα–CH), 56.59 (CH, Thrα–CH), 70.43 (CH, Thrβ–CH), 109.98 (quat., Trp–C4), 110.93 (quat., Trp–C4), 111.85 (CH, Trp–C9), 118.54 (CH, Trp–C6 and C7), 118.77 (CH, Trp–C6 and C7), 121.33 (CH, Trp–C8), 121.39 (CH, Trp–C8), 123.66 (CH, Trp–C12), 126.90 (CH, Ph), 127.51 (quat., Trp–C5), 127.59 (quat., Trp–C5), 128.75 (CH, Ph), 129.48 (CH, Ph), 136.47 (quat., Trp–C10), 136.85 (quat., PA–Ph), 169.70 (quat., β–Ala–CON), 171.30 (quat., Thr–CON), 171.45 (quat., PhCH2CON), 171.52 (quat., Trp–CON), 171.76 (quat., Trp–CON); m/z (EI) 663.2918 (MH+, C37H39N6O6 requires 663.2926), 663 (MH+, 21%), 682 (30), 685 (MNa+, 100%), 686 (40), 687 (9) and 701 (42). (* 1H peaks for PhCH2CON are not included as they are obscured by DMSO-d6.)
1 v/v, 139 mL) at 0 °C, BOPCl (0.13 g, 0.51 mmol) and DMAP (0.11 g, 0.92 mmol) were added, and the reaction was stirred for 19 h. 1 M HCl solution (30 mL) was added and the aqueous layer was extracted with dichloromethane (3 × 80 mL). The combined organic extracts were dried over anhydrous Na2SO4, filtered and concentrated in vacuo. Purification by semi-preparative RP–HPLC (using a linear gradient of 40% B to 70% B) yielded the title compound (8% from H2N-L-Trp-2-ClTrt-resin) as an off-white amorphous solid in >99% purity according to analytical RP-HPLC; Rt 10.77 min (XTerra C18, 4.6 × 150 mm, 30% to 75% B over 15 min, 1 mL min−1); mp 150.2–156.4 °C; vmax(film)/cm−1 3315, 1736, 1649, 1514, 1165, 1060, 743 and 697; [α]20D −36.9 (c 0.52 in MeOH); δH(300 MHz; DMSO-d6)* 1.03 (3H, d, Thrβ–CH3), 2.28–2.44 (2H, m, β–Ala–CH2), 2.74–2.95 (3H, m, Trpβ–CH2), 3.12–3.17 (1H, m, Trpβ–CH2), 3.55–3.59 (2H, m, β–Ala–CH2), 4.30–4.34 (1H, m, Trpα–CH), 4.44–4.48 (1H, m, Thrα–CH), 4.53–4.57 (1H, m, Trpα–CH), 5.41–5.43 (1H, m, Thrβ–CH), 6.91–6.95 (6H, m, Trp–H7, Trp–H8, Trp–H12), 7.22–7.35 (8H, m, β–Ala–NH, Trp–H9, PA–Ph), 7.41 (1H, d, J 7.8, Trp–H6), 7.46 (1H, d, J 7.8, Trp–H6), 7.82 (1H, d, J 7.8, Thr–CONH), 8.44–8.59 (2H, m, Trp–CONH), 10.69 (2H, s, Trp–H11); δC(75 MHz, DMSO-d6) 16.95 (CH3, Thrβ–CH3), 26.45 (CH2, Trpβ–CH2), 27.04 (CH2, Trpβ–CH2), 34.77 (CH2, β-Ala–CH2), 35.21 (CH2, β-Ala–CH2), 42.56 (CH2, PhCH2CON), 53.70 (CH, Trpα–CH), 55.32 (CH, Trpα–CH), 56.59 (CH, Thrα–CH), 70.43 (CH, Thrβ–CH), 109.98 (quat., Trp–C4), 110.93 (quat., Trp–C4), 111.85 (CH, Trp–C9), 118.54 (CH, Trp–C6 and C7), 118.77 (CH, Trp–C6 and C7), 121.33 (CH, Trp–C8), 121.39 (CH, Trp–C8), 123.66 (CH, Trp–C12), 126.90 (CH, Ph), 127.51 (quat., Trp–C5), 127.59 (quat., Trp–C5), 128.75 (CH, Ph), 129.48 (CH, Ph), 136.47 (quat., Trp–C10), 136.85 (quat., PA–Ph), 169.70 (quat., β–Ala–CON), 171.30 (quat., Thr–CON), 171.45 (quat., PhCH2CON), 171.52 (quat., Trp–CON), 171.76 (quat., Trp–CON); m/z (EI) 663.2918 (MH+, C37H39N6O6 requires 663.2926), 663 (MH+, 21%), 682 (30), 685 (MNa+, 100%), 686 (40), 687 (9) and 701 (42). (* 1H peaks for PhCH2CON are not included as they are obscured by DMSO-d6.)
4.4 Synthesis of PA-L-[Thr-L-Trp-D-Trp-β-Ala] 12
The title compound [4% from resin 13 (0.39 mmol g−1) (0.25 g, 0.1 mmol)] was obtained as a colourless amorphous solid in >99% purity according to analytical RP-HPLC; Rt 10.70 min (XTerra C18, 4.6 × 150 mm, 30% to 75% B over 15 min, 1 mL min−1); mp 181.9–184.6 °C; vmax(film)/cm−1 3276, 1739, 1634, 1547, 1261, 1233, 1187 and 742; [α]20D +61.0 (c 0.58 in MeOH); δH(300 MHz; DMSO-d6) 1.04 (3H, d, J 6.0, Thrβ–CH3), 2.37–2.49 (2H, m, β–Ala–CH2), 2.82–2.95 (3H, m, Trpβ–CH2), 3.16–3.21 (1H, m, Trpβ–CH2), 3.39 (2H, m, β–Ala–CH2), 3.54 (1H, d, J 14.1, PhCH2CON), 3.65 (1H, d, J 14.1, PhCH2CON), 4.17 (1H, ddd, J 10.2, 6.6 and 3.6, Trpα–CH), 4.52 (1H, q, J 7.2, Trpα–CH), 4.62–4.65 (1H, m, Thrα–CH), 5.09–5.11 (1H, m, Thrβ–CH), 6.93–7.09 (6H, m, Trp–H7, Trp–H8, Trp–H12), 7.17–7.42 (8H, m, β–Ala–NH, Trp–H9, PA–Ph), 7.48 (1H, d, J 7.8, Trp–H6), 7.54 (1H, d, J 7.5, Trp–H6), 8.13 (1H, d, J 6.9, Thr–CONH), 8.76 (1H, d, J 6.9, Trp–CONH), 8.82 (1H, d, J 6.6, Trp–CONH), 10.63 (1H, s, Trp–H11), 10.72 (1H, s, Trp–H11); δC(75 MHz; DMSO-d6) 16.70 (CH3, Thrβ–CH3), 26.25 (CH2, Trpβ–CH2), 34.48 (CH2, β–Ala–CH2), 35.13 (CH2, β–Ala–CH2), 42.17 (CH2, PhCH2CON), 54.44 (CH, Trpα–CH), 54.53 (CH, Thrα–CH), 55.07 (CH, Trpα–CH), 72.54 (CH, Thrβ–CH), 109.82 (quat., Trp–C4), 111.17 (quat., Trp–C4), 111.84 (CH, Trp–C9), 111.90 (CH, Trp–C9), 118.49 (CH, Trp–C6), 118.81 (CH, Trp–C6 and C7), 121.38 (CH, Trp–C8), 121.53 (CH, Trp–C8), 123.70 (CH, Trp–C12), 123.96 (CH, Trp–C12), 126.78 (CH, Ph), 127.40 (quat., Trp–C5), 127.49 (quat., Trp–C5), 128.64 (CH, Ph), 129.55 (CH, Ph), 136.45 (quat., Trp–C10), 136.54 (quat., Trp–C10), 136.83 (quat., PA–Ph), 169.78 (quat., β–Ala–CON), 170.83 (quat., Thr–CON), 171.20 (quat., PhCH2CON), 171.61 (quat., Trp–CON), 172.58 (quat., Trp–CON); m/z (EI) 663.2913 (MH+, C37H39N6O6 requires 663.2926), 663 (MH+, 20%), 685 (MNa+, 100%), 686 (32), 687 (8) and 701 (35).
4.5 Synthesis of PA-L-[Thr-L-Trp-L-Trp-β-Ala]
The title compound (6% from H2N-L-Trp-2-ClTrt-resin) was obtained as an off-white amorphous solid in >99% purity according to analytical RP-HPLC; Rt 10.46 min (XTerra C18, 4.6 × 150 mm, 30% to 75% B over 15 min, 1 mL min−1); mp 162.6–167.4 °C; vmax(film)/cm−1 3324, 1727, 1649, 1522, 1457, 1177, 1069, 742 and 703; [α]20D −30.7 (c 0.81 in MeOH); δH(300 MHz; DMSO-d6) 1.13 (3H, d, J 6.3, Thrβ–CH3), 2.29–2.37 (1H, m, β-Ala–CH2), 2.60–2.70 (1H, m, β–Ala–CH2), 2.93–3.03 (2H, m, Trpβ–CH2), 3.05–3.10 (1H, m, Trpβ–CH2), 3.24–3.35 (1H, m, Trpβ–CH2), 3.36–3.41 (2H, m, β–Ala–CH2), 3.61 (2H, m, PhCH2CON), 4.15–4.28 (2H, m, Trpα–CH), 4.61–4.65 (1H, m, Thrα–CH), 5.03–5.06 (1H, qd, J 6.3 and 1.5, Thrβ–CH), 6.53 (2H, s, Trp–H8), 6.83 (2H, s, Trp–H12), 7.02–7.08 (1H, m, Trp–H9), 7.12–7.17 (1H, m, Trp–H9), 7.19–7.38 (7H, m, Trp–H7 and PA–Ph), 7.43–7.47 (2H, m, Trp–H6); δC(75 MHz; DMSO-d6) 16.16 (CH3, Thrβ–CH3), 24.48 (CH2, Trpβ–CH2), 25.80 (CH2, Trpβ–CH2), 34.28 (CH2, β–Ala–CH2), 34.99 (CH2, β–Ala–CH2), 42.85 (CH2, PhCH2CON), 54.61 (CH, Thrα–CH), 55.22 (CH, Trpα–CH), 55.94 (CH, Trpα–CH), 72.25 (CH, Thrβ–CH), 108.70 (quat., Trp–C4), 110.68 (quat., Trp–C4), 111.00 (CH, Trp–C9), 111.15 (CH, Trp–C9), 117.94 (CH, Trp–C6), 118.09 (CH, Trp–C7), 118.72 (CH, Trp–C6), 118.95 (CH, Trp–C7), 121.32 (CH, Trp–C8), 121.62 (CH, Trp–C8), 122.90 (CH, Trp–C12), 123.20 (CH, Trp–C12), 126.71 (quat., Trp–C5), 127.12 (quat., Trp–C5), 127.21 (CH, Ph), 128.61 (CH, Ph), 129.03 (CH, Ph), 134.24 (quat., PA–Ph), 136.00 (quat., Trp–C10), 136.12 (quat., Trp–C10), 169.96 (quat., β–Ala–CON), 171.06 (quat., Thr–CON), 171.42 (quat., PhCH2CON), 171.99 (quat., Trp–CON), 172.08 (quat., Trp–CON); m/z (EI) 663.2913 (MH+, C37H39N6O6 requires 663.2926), 663 (MH+, 20%), 685 (MNa+, 100%), 686 (32), 687 (8) and 701 (35).
4.6 Synthesis of PA-L-[Thr-D-Trp-D-Trp-β-Ala]
The title compound [8% from resin 13 (0.39 mmol g−1) (0.25 g, 0.1 mmol)] was obtained as an off-white amorphous solid in >90% purity according to analytical RP-HPLC; Rt 10.53 min (XTerra C18, 4.6 × 150 mm, 30% to 75% B over 15 min, 1 mL min−1); mp 155.2–159.2 °C; vmax(film)/cm−1 3315, 1727, 1657, 1527, 1457, 1166, 1061, 743 and 698; [α]20D +51.1 (c 0.99 in MeOH); δH(300 MHz; DMSO-d6) 0.92 (3H, d, J 6.6, Thrβ–CH3), 2.33–2.36 (2H, m, β-Ala–CH2), 2.88–3.03 (2H, m, Trpβ–CH2), 3.05–3.19 (2H, m, Trpβ–CH2), 3.42–3.54 (2H, m, β–Ala–CH2), 3.78–3.83 (2H, m, PhCH2CON), 4.35–4.44 (3H, m, Trpα–CH and Thrα–CH), 5.48–5.54 (1H, qd, J 6.6 and 4.5, Thrβ–CH), 6.40 (2H, s, Trp–H8), 6.80 (2H, s, Trp–H12), 7.04–7.10 (2H, m, Trp–H9), 7.12–7.21 (2H, m, Trp–H6), 7.27–7.42 (7H, m, Trp–H7 and PA–Ph); δC(75 MHz; DMSO-d6) 16.09 (CH3, Thrβ–CH3), 25.26 (CH2, Trpβ–CH2), 26.19 (CH2, Trpβ–CH2), 34.57 (CH2, β–Ala–CH2), 35.36 (CH2, β–Ala–CH2), 42.84 (CH2, PhCH2CON), 54.13 (CH, Trpα–CH), 54.94 (CH, Trpα–CH), 56.11 (CH, Thrα–CH), 69.50 (CH, Thrβ–CH), 108.22 (quat., Trp–C4), 111.17 (CH, Trp–C9), 111.32 (CH, Trp–C9), 117.74 (CH, Trp–C6), 118.43 (CH, Trp–C6), 118.93 (CH, Trp–C7), 119.47 (CH, Trp–C7), 121.61 (CH, Trp–C8), 121.88 (CH, Trp–C8), 122.82 (CH, Trp–C12), 123.44 (CH, Trp–C12), 126.94 (quat., Trp–C5), 127.21 (CH, Ph), 127.38 (quat., Trp–C5), 128.72 (CH, Ph), 128.81 (CH, Ph), 134.66 (quat., PA–Ph), 135.98 (quat., Trp–C10), 136.05 (quat., Trp–C10), 169.99 (quat., β–Ala–CON), 171.39 (quat., Thr–CON and PhCH2CON), 171.89 (quat., Trp–CON), 172.63 (quat., Trp–CON); m/z (EI) 663.2920 (MH+, C37H39N6O6 requires 663.2926), 663 (MH+, 100%), 664 (40), 665 (10), 666 (2), 685 (MNa+, 60%), 686 (32) and 687 (8).
Acknowledgements
We thank the Maurice Wilkins Centre for Molecular Discovery for financial support.
Notes and references
- G. Lang, T. Kalvelage, A. Peters, J. Wiese and J. F. Imhoff, J. Nat. Prod., 2008, 71, 1074–1077 CrossRef CAS.
- J. M. Crawford, R. Kontnik and J. Clardy, Curr. Biol., 2010, 20, 69–74 CrossRef CAS.
- J. L. Norelli, A. L. Jones and H. S. Aldwinckle, Plant Dis., 2003, 87, 756–765 Search PubMed.
- E. Badosa, Peptides, 2007, 28, 2276–2285 CrossRef CAS.
- J. E. Loper, Plant Dis., 1991, 75, 287–290 Search PubMed.
- M. Quibell, L. C. Packman and T. Johnson, J. Am. Chem. Soc., 1995, 117, 11656–11668 CrossRef CAS.
- M. Harre, K. Nickisch and U. Tilstam, React. Funct. Polym., 1999, 41, 111–114 CrossRef CAS.
-
K. Barlos and D. Gatos, in Fmoc Solid Phase Peptide Synthesis, ed. W. C. Chan and P. D. White, Information Press, Oxford, U. K., 2000, ch. 9, pp. 216 Search PubMed.
- K. Barlos, O. Chatzi and D. Gatos, Int. J. Peptide Protein Res., 1991, 37, 513–520 CAS.
- G. P. Nora, M. J. Miller and U. Möllmann, Bioorg. Med. Chem. Lett., 2006, 16, 3966–3970 CrossRef CAS.
- M. Stawikowski and P. Cudic, Tetrahedron Lett., 2006, 47, 8587–8590 CrossRef CAS.
- I. Dhimitruka and Jr. J. SantaLucia, Org. Lett., 2006, 8, 47–50 CrossRef CAS.
- A. K. Ghosh and C. Liu, Org. Lett., 2001, 3, 635–638 CrossRef CAS.
-
W. C. Chan and P. D. White, in Fmoc Solid Phase Peptide Synthesis, ed. W. C. Chan and P. D. White, Information Press, Oxford, U. K., 2000, ch. 9, pp. 62 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The NMR (1H and 13C) spectra of PA-L-Thr-OBn, compound 9, isolated xenematide and the four diastereomers of xenematide, and the HPLC chromatograms of peptides 8, 10 (after cleavage from resin) and the four diastereomers of xenematide are provided. See DOI: 10.1039/c0ob00315h |
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5), 1 h.
2.5), 1 h.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5), intramolecular cyclization was carried out using BOPCl and DMAP13 to give the diastereomer PA-L-[Thr-D-Trp-L-Trp-β-Ala] 11 (Scheme 4).
2.5), intramolecular cyclization was carried out using BOPCl and DMAP13 to give the diastereomer PA-L-[Thr-D-Trp-L-Trp-β-Ala] 11 (Scheme 4).![Synthesis of PA-l-[Thr-d-Trp-l-Trp-β-Ala] 11. Reagents, conditions and yields: i) Boc-β-Ala-OH (20 equiv.), BzCl (20 equiv.), Et3N (40 equiv.), CH2Cl2, rt, 18 h; ii) TFA : H2O :TIPS (95 : 2.5 : 2.5), 1 h; iii) BOPCl, DMAP, CH2Cl2–MeOH, 0 °C to rt, 19 h, 8% from compound 5.](/image/article/2011/OB/c0ob00315h/c0ob00315h-s4.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5), 1 h; iii) BOPCl, DMAP, CH2Cl2–MeOH, 0 °C to rt, 19 h, 8% from compound 5.
2.5), 1 h; iii) BOPCl, DMAP, CH2Cl2–MeOH, 0 °C to rt, 19 h, 8% from compound 5.![Synthesis of PA-l-[Thr-l-Trp-d-Trp-β-Ala] 12. Reagents, conditions and yields: i) Fmoc-l-Trp(Boc)-OH (3 equiv.), HBTU, DIPEA, DMF, rt, 45 min; ii) 20% piperidine/DMF, rt; iii) PA-l-Thr-OH (3 equiv.), HBTU, DIPEA, DMF, rt, 45 min; iv) Boc-β-Ala-OH (20 equiv.), BzCl (20 equiv.), Et3N (40 equiv.), CH2Cl2, rt, 18 h; v) TFA : H2O : TIPS (95 : 2.5 : 2.5), 1 h; vi) BOPCl, DMAP, CH2Cl2–MeOH, 0 °C to rt, 19 h, 4% from compound 13.](/image/article/2011/OB/c0ob00315h/c0ob00315h-s5.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5), 1 h; vi) BOPCl, DMAP, CH2Cl2–MeOH, 0 °C to rt, 19 h, 4% from compound 13.
2.5), 1 h; vi) BOPCl, DMAP, CH2Cl2–MeOH, 0 °C to rt, 19 h, 4% from compound 13.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EtOAc–hexane); mp 150.5–152.8 °C; vmax(film)/cm−1 3469, 3349, 1705, 1648, 1529, 1279, 1001, 725 and 693; [α]20D −3.6 (c 1.11 in CDCl3); δH(300 MHz; CDCl3) 1.04 (3H, d, J 6.0, Thrβ–CH3), 3.55 (2H, s, PhCH2CON), 4.25 (1H, dq, J 3.0 and 6.0, Thrβ–CH), 4.48–4.52 (1H, m, Thrα–CH), 5.07 (2H, s, PhCH2CO2), 6.88 (1H, d, CONHCH), 7.17–7.27 (10H, m, Ph); δC(75 MHz; CDCl3) 19.6 (CH3, Thrβ–CH3), 42.95 (CH2, PhCH2CON), 43.0 (CH2, PhCH2CON), 57.6 (CH, Thrα–CH), 57.65 (CH, Thrα–CH), 67.1 (CH2, PhCH2CO2), 67.2 (CH, Thrβ–CH), 126.9 (CH, Ph), 127.9 (CH, Ph), 128.1 (CH, Ph), 128.2 (CH, Ph), 128.4 (CH, Ph), 128.6 (CH, Ph), 129.0 (CH, Ph), 129.6 (CH, Ph), 132.75 (quat., Ph), 134.4 (quat., Ph), 135.1 (quat., Ph), 170.6 (quat., CHCO2CH2), 172.1 (quat., CH2CON), 172.2 (quat., CH2CON); m/z (EI) 328.1531 (MH+, C19H22NO4 requires 328.1543), 328 (MH+, 6%), 350 (MNa+, 100%), 351 (20) and 352 (2).
1 EtOAc–hexane); mp 150.5–152.8 °C; vmax(film)/cm−1 3469, 3349, 1705, 1648, 1529, 1279, 1001, 725 and 693; [α]20D −3.6 (c 1.11 in CDCl3); δH(300 MHz; CDCl3) 1.04 (3H, d, J 6.0, Thrβ–CH3), 3.55 (2H, s, PhCH2CON), 4.25 (1H, dq, J 3.0 and 6.0, Thrβ–CH), 4.48–4.52 (1H, m, Thrα–CH), 5.07 (2H, s, PhCH2CO2), 6.88 (1H, d, CONHCH), 7.17–7.27 (10H, m, Ph); δC(75 MHz; CDCl3) 19.6 (CH3, Thrβ–CH3), 42.95 (CH2, PhCH2CON), 43.0 (CH2, PhCH2CON), 57.6 (CH, Thrα–CH), 57.65 (CH, Thrα–CH), 67.1 (CH2, PhCH2CO2), 67.2 (CH, Thrβ–CH), 126.9 (CH, Ph), 127.9 (CH, Ph), 128.1 (CH, Ph), 128.2 (CH, Ph), 128.4 (CH, Ph), 128.6 (CH, Ph), 129.0 (CH, Ph), 129.6 (CH, Ph), 132.75 (quat., Ph), 134.4 (quat., Ph), 135.1 (quat., Ph), 170.6 (quat., CHCO2CH2), 172.1 (quat., CH2CON), 172.2 (quat., CH2CON); m/z (EI) 328.1531 (MH+, C19H22NO4 requires 328.1543), 328 (MH+, 6%), 350 (MNa+, 100%), 351 (20) and 352 (2).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to give compound 9 (1.85 g, 71%) as a yellow oil; Rf 0.39 (1
2) to give compound 9 (1.85 g, 71%) as a yellow oil; Rf 0.39 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 EtOAc–hexane); vmax(film)/cm−1 3302, 1739, 1663, 1519, 1164, 731 and 697; [α]20D +17.3 (c 2.61 in CDCl3); δH(300 MHz; CDCl3) 1.17 (3H, d, J 6.0, Thrβ–CH3), 1.44 [9H, s, C(CH3)3], 2.21–2.30 (2H, m, CO2CH2CH2NH), 3.16–3.27 (2H, m, CO2CH2CH2NH), 3.67 (2H, s, PhCH2CON), 4.79 (1H, d, J 9.0, Thrα–CH), 5.09 (2H, d, PhCH2CO2), 5.38 (1H, d, J 3.0, Thrβ–CH), 7.26–7.37 (10H, m, Ph); δC(75 MHz; CDCl3) 16.9 (CH3, Thrβ–CH3), 28.3 [CH3, C(CH3)3], 34.6 (CH2, β–Ala–CH2), 35.9 (CH2, β–Ala–CH2), 43.3 (CH2, PhCH2CON), 55.5 (CH, Thrα–C), 67.5 (CH2, PhCH2CO2), 70.6 (CH, Thrβ–C), 79.4 [quat., C(CH3)3], 127.3 (CH, Ph), 128.3 (CH, Ph), 128.5 (CH, Ph), 128.6 (CH, Ph), 128.8 (CH, Ph), 129.3 (CH, Ph), 129.9 (CH, Ph), 133.1 (CH, Ph), 134.5 (quat., Ph), 134.9 (quat., Ph), 155.8 (quat., NCO2C), 169.5 (quat., CHCO2CH2, CHCO2CH2), 170.5 (quat., CH2CON); m/z (EI) 521.2256 (MNa+, C27H34N2NaO7 requires 521.2258), 521 (MNa+, 100%), 522 (48), 523 (10), 537 (20) and 538 (4).
2 EtOAc–hexane); vmax(film)/cm−1 3302, 1739, 1663, 1519, 1164, 731 and 697; [α]20D +17.3 (c 2.61 in CDCl3); δH(300 MHz; CDCl3) 1.17 (3H, d, J 6.0, Thrβ–CH3), 1.44 [9H, s, C(CH3)3], 2.21–2.30 (2H, m, CO2CH2CH2NH), 3.16–3.27 (2H, m, CO2CH2CH2NH), 3.67 (2H, s, PhCH2CON), 4.79 (1H, d, J 9.0, Thrα–CH), 5.09 (2H, d, PhCH2CO2), 5.38 (1H, d, J 3.0, Thrβ–CH), 7.26–7.37 (10H, m, Ph); δC(75 MHz; CDCl3) 16.9 (CH3, Thrβ–CH3), 28.3 [CH3, C(CH3)3], 34.6 (CH2, β–Ala–CH2), 35.9 (CH2, β–Ala–CH2), 43.3 (CH2, PhCH2CON), 55.5 (CH, Thrα–C), 67.5 (CH2, PhCH2CO2), 70.6 (CH, Thrβ–C), 79.4 [quat., C(CH3)3], 127.3 (CH, Ph), 128.3 (CH, Ph), 128.5 (CH, Ph), 128.6 (CH, Ph), 128.8 (CH, Ph), 129.3 (CH, Ph), 129.9 (CH, Ph), 133.1 (CH, Ph), 134.5 (quat., Ph), 134.9 (quat., Ph), 155.8 (quat., NCO2C), 169.5 (quat., CHCO2CH2, CHCO2CH2), 170.5 (quat., CH2CON); m/z (EI) 521.2256 (MNa+, C27H34N2NaO7 requires 521.2258), 521 (MNa+, 100%), 522 (48), 523 (10), 537 (20) and 538 (4).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 4 mL) dropwise, and the reaction was gently stirred at room temperature for 3 h. The resin was washed with DMF (2 × 1 mL), dichloromethane (3 × 1 mL) and then dried for 10 min. To this resin was added a solution of Fmoc-D-Trp(Boc)-OH (0.12 g, 0.2 mmol) and DIPEA (92.1 μL, 0.5 mmol) in dry dichloromethane (2 mL), and the reaction was gently stirred at room temperature for 30 min. After washing with DMF (2 × 1 mL), a solution of CH2Cl2/MeOH/DIPEA (80
1 v/v, 4 mL) dropwise, and the reaction was gently stirred at room temperature for 3 h. The resin was washed with DMF (2 × 1 mL), dichloromethane (3 × 1 mL) and then dried for 10 min. To this resin was added a solution of Fmoc-D-Trp(Boc)-OH (0.12 g, 0.2 mmol) and DIPEA (92.1 μL, 0.5 mmol) in dry dichloromethane (2 mL), and the reaction was gently stirred at room temperature for 30 min. After washing with DMF (2 × 1 mL), a solution of CH2Cl2/MeOH/DIPEA (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v, 10 mL) was added, the reaction was stirred for 10 min and repeated. After washing with DMF (3 × 1 mL), a 20% piperidine/DMF solution (v/v, 10 mL) was added and the reaction was stirred for 3 min and then repeated for 20 min. The resin was washed with DMF (6 × 1 mL), iPrOH (3 × 1 mL), hexane (4 × 1 mL), air dried for 15 min and then dried under N2. The loading of Fmoc-D-Trp(Boc)-OH was found to be 0.39 mmol g−1 (theoretical loading = 0.58 mmol g−1, 67%) using the Fmoc assay.14
5 v/v, 10 mL) was added, the reaction was stirred for 10 min and repeated. After washing with DMF (3 × 1 mL), a 20% piperidine/DMF solution (v/v, 10 mL) was added and the reaction was stirred for 3 min and then repeated for 20 min. The resin was washed with DMF (6 × 1 mL), iPrOH (3 × 1 mL), hexane (4 × 1 mL), air dried for 15 min and then dried under N2. The loading of Fmoc-D-Trp(Boc)-OH was found to be 0.39 mmol g−1 (theoretical loading = 0.58 mmol g−1, 67%) using the Fmoc assay.14
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 v/v, 10 mL) and the reaction was agitated for 1 h. The solution was filtered and concentrated in vacuo. To the resultant yellow residue (69.5 mg, 0.1 mmol) was dissolved in dichloromethane–methanol (4
2.5 v/v, 10 mL) and the reaction was agitated for 1 h. The solution was filtered and concentrated in vacuo. To the resultant yellow residue (69.5 mg, 0.1 mmol) was dissolved in dichloromethane–methanol (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 139 mL) at 0 °C, BOPCl (0.13 g, 0.51 mmol) and DMAP (0.11 g, 0.92 mmol) were added, and the reaction was stirred for 19 h. 1 M HCl solution (30 mL) was added and the aqueous layer was extracted with dichloromethane (3 × 80 mL). The combined organic extracts were dried over anhydrous Na2SO4, filtered and concentrated in vacuo. Purification by semi-preparative RP–HPLC (using a linear gradient of 40% B to 70% B) yielded the title compound (8% from H2N-L-Trp-2-ClTrt-resin) as an off-white amorphous solid in >99% purity according to analytical RP-HPLC; Rt 10.77 min (XTerra C18, 4.6 × 150 mm, 30% to 75% B over 15 min, 1 mL min−1); mp 150.2–156.4 °C; vmax(film)/cm−1 3315, 1736, 1649, 1514, 1165, 1060, 743 and 697; [α]20D −36.9 (c 0.52 in MeOH); δH(300 MHz; DMSO-d6)* 1.03 (3H, d, Thrβ–CH3), 2.28–2.44 (2H, m, β–Ala–CH2), 2.74–2.95 (3H, m, Trpβ–CH2), 3.12–3.17 (1H, m, Trpβ–CH2), 3.55–3.59 (2H, m, β–Ala–CH2), 4.30–4.34 (1H, m, Trpα–CH), 4.44–4.48 (1H, m, Thrα–CH), 4.53–4.57 (1H, m, Trpα–CH), 5.41–5.43 (1H, m, Thrβ–CH), 6.91–6.95 (6H, m, Trp–H7, Trp–H8, Trp–H12), 7.22–7.35 (8H, m, β–Ala–NH, Trp–H9, PA–Ph), 7.41 (1H, d, J 7.8, Trp–H6), 7.46 (1H, d, J 7.8, Trp–H6), 7.82 (1H, d, J 7.8, Thr–CONH), 8.44–8.59 (2H, m, Trp–CONH), 10.69 (2H, s, Trp–H11); δC(75 MHz, DMSO-d6) 16.95 (CH3, Thrβ–CH3), 26.45 (CH2, Trpβ–CH2), 27.04 (CH2, Trpβ–CH2), 34.77 (CH2, β-Ala–CH2), 35.21 (CH2, β-Ala–CH2), 42.56 (CH2, PhCH2CON), 53.70 (CH, Trpα–CH), 55.32 (CH, Trpα–CH), 56.59 (CH, Thrα–CH), 70.43 (CH, Thrβ–CH), 109.98 (quat., Trp–C4), 110.93 (quat., Trp–C4), 111.85 (CH, Trp–C9), 118.54 (CH, Trp–C6 and C7), 118.77 (CH, Trp–C6 and C7), 121.33 (CH, Trp–C8), 121.39 (CH, Trp–C8), 123.66 (CH, Trp–C12), 126.90 (CH, Ph), 127.51 (quat., Trp–C5), 127.59 (quat., Trp–C5), 128.75 (CH, Ph), 129.48 (CH, Ph), 136.47 (quat., Trp–C10), 136.85 (quat., PA–Ph), 169.70 (quat., β–Ala–CON), 171.30 (quat., Thr–CON), 171.45 (quat., PhCH2CON), 171.52 (quat., Trp–CON), 171.76 (quat., Trp–CON); m/z (EI) 663.2918 (MH+, C37H39N6O6 requires 663.2926), 663 (MH+, 21%), 682 (30), 685 (MNa+, 100%), 686 (40), 687 (9) and 701 (42). (* 1H peaks for PhCH2CON are not included as they are obscured by DMSO-d6.)
1 v/v, 139 mL) at 0 °C, BOPCl (0.13 g, 0.51 mmol) and DMAP (0.11 g, 0.92 mmol) were added, and the reaction was stirred for 19 h. 1 M HCl solution (30 mL) was added and the aqueous layer was extracted with dichloromethane (3 × 80 mL). The combined organic extracts were dried over anhydrous Na2SO4, filtered and concentrated in vacuo. Purification by semi-preparative RP–HPLC (using a linear gradient of 40% B to 70% B) yielded the title compound (8% from H2N-L-Trp-2-ClTrt-resin) as an off-white amorphous solid in >99% purity according to analytical RP-HPLC; Rt 10.77 min (XTerra C18, 4.6 × 150 mm, 30% to 75% B over 15 min, 1 mL min−1); mp 150.2–156.4 °C; vmax(film)/cm−1 3315, 1736, 1649, 1514, 1165, 1060, 743 and 697; [α]20D −36.9 (c 0.52 in MeOH); δH(300 MHz; DMSO-d6)* 1.03 (3H, d, Thrβ–CH3), 2.28–2.44 (2H, m, β–Ala–CH2), 2.74–2.95 (3H, m, Trpβ–CH2), 3.12–3.17 (1H, m, Trpβ–CH2), 3.55–3.59 (2H, m, β–Ala–CH2), 4.30–4.34 (1H, m, Trpα–CH), 4.44–4.48 (1H, m, Thrα–CH), 4.53–4.57 (1H, m, Trpα–CH), 5.41–5.43 (1H, m, Thrβ–CH), 6.91–6.95 (6H, m, Trp–H7, Trp–H8, Trp–H12), 7.22–7.35 (8H, m, β–Ala–NH, Trp–H9, PA–Ph), 7.41 (1H, d, J 7.8, Trp–H6), 7.46 (1H, d, J 7.8, Trp–H6), 7.82 (1H, d, J 7.8, Thr–CONH), 8.44–8.59 (2H, m, Trp–CONH), 10.69 (2H, s, Trp–H11); δC(75 MHz, DMSO-d6) 16.95 (CH3, Thrβ–CH3), 26.45 (CH2, Trpβ–CH2), 27.04 (CH2, Trpβ–CH2), 34.77 (CH2, β-Ala–CH2), 35.21 (CH2, β-Ala–CH2), 42.56 (CH2, PhCH2CON), 53.70 (CH, Trpα–CH), 55.32 (CH, Trpα–CH), 56.59 (CH, Thrα–CH), 70.43 (CH, Thrβ–CH), 109.98 (quat., Trp–C4), 110.93 (quat., Trp–C4), 111.85 (CH, Trp–C9), 118.54 (CH, Trp–C6 and C7), 118.77 (CH, Trp–C6 and C7), 121.33 (CH, Trp–C8), 121.39 (CH, Trp–C8), 123.66 (CH, Trp–C12), 126.90 (CH, Ph), 127.51 (quat., Trp–C5), 127.59 (quat., Trp–C5), 128.75 (CH, Ph), 129.48 (CH, Ph), 136.47 (quat., Trp–C10), 136.85 (quat., PA–Ph), 169.70 (quat., β–Ala–CON), 171.30 (quat., Thr–CON), 171.45 (quat., PhCH2CON), 171.52 (quat., Trp–CON), 171.76 (quat., Trp–CON); m/z (EI) 663.2918 (MH+, C37H39N6O6 requires 663.2926), 663 (MH+, 21%), 682 (30), 685 (MNa+, 100%), 686 (40), 687 (9) and 701 (42). (* 1H peaks for PhCH2CON are not included as they are obscured by DMSO-d6.)
