Synthesis of highly porous borazine-linked polymers and their application to H2, CO2, and CH4 storage†
Karl T.
Jackson
,
Mohammad G.
Rabbani
,
Thomas E.
Reich
and
Hani M.
El-Kaderi
*
Department of Chemistry, Virginia Commonwealth University, Richmond, VA 23284-2006, United States. E-mail: helkaderi@vcu.edu; Fax: +1 804 828 8599; Tel: +1 804 828 7505
First published on 17th October 2011
Abstract
The synthesis of highly porous borazine-linked polymers (BLPs) and their gas uptakes are reported. BLPs exhibit high surface areas up to 2866 m2 g−1 and can store significant amounts of H2 (1.93 wt%) and CO2 (12.8 wt%) at 77 K and 273 K, respectively at 1.0 bar with respective isosteric heats of adsorption of 6.0 and 25.2 kJ mol−1.
Recently there has been great interest in the design and synthesis of highly porous organic architectures due to their multifaceted potential use in applications that include storage, separation, conductivity, and catalysis.1 The chemical composition, physical and textural properties are dictated during synthesis that allow for materials with enhanced properties relevant to their respective applications. With the exception of microcrystalline covalent-organic frameworks (COFs),2,3 these polymeric materials are amorphous yet can possess considerable porosity and well-defined cavities which render them highly attractive especially in adsorptive gas storage.4 Such desirable traits are imparted into organic materials through the use of rigid building blocks that direct the growth of polymer networks without the aid of templating agents.1–3 In addition to customized porosity, polymerization processes can lead to pore wall functionalization that significantly enhance gas uptake and selectivity as we have demonstrated recently for benzimidazole-linked polymers.5 Alternative methods for improved gas uptake (i.e.hydrogen) by porous architectures can also be accessed by the use of polarizable building units that increase hydrogen-framework interactions.6 Along this line, we sought after the inclusion of borazine (B3N3) as a functionalized and polarizable building block into porous organic polymers.7Borazine is isostructural to the boroxine units found in COFs prepared by boronic acid self-condensation reactions2 and has been mainly used for the fabrication of BN-based ceramics or in organic optoelectronics.8–10 However, up to date, the use of borazine for the preparation of porous polymers for gas storage remains fairly undeveloped.
We report herein on the synthesis and characterization of a new class of highly porous borazine-linked polymers and investigate their performance in gas (H2, CO2, CH4) storage application under low pressure and cryogenic conditions. The synthesis of BLP-1(H) and BLP-12(H) was performed by thermolysis of 1,4-phenylenediamine and tetra-(4-aminophenyl)methane borane adducts in monoglyme to afford the corresponding polymers as white powders in good yields (Scheme 1).
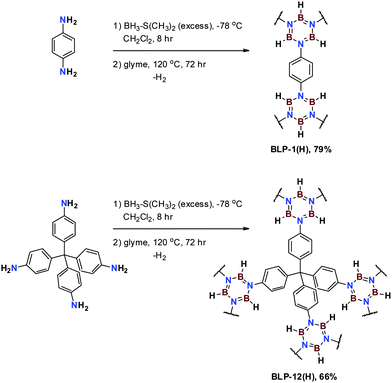 | ||
| Scheme 1 Synthesis of BLP-1(H) and BLP-12(H) from in situthermal decomposition of arylamine-borane adducts. | ||
BLPs were isolated under an inert atmosphere followed by degassing at 120 °C/1.0 × 10−5 torr for 16 h. Scanning electron microscopy (SEM) on as-prepared materials revealed a homogeneous morphology of spherical particles ∼150 nm for BLP-1(H), and platelet particles ∼200 nm for BLP-12(H) indicating phase purity (Fig. S13–S14, ESI). Powder X-ray diffraction studies on as-prepared and activated materials indicated that both polymers are amorphous. The chemical composition and connectivity of the building units were investigated by spectral and analytical methods which included FT-IR, solid-state 11B and 13C CP-MAS, and elemental analysis. The formation of the borazine ring was first established by FT-IR studies (Fig. S1–S8, ESI) which revealed a significant depletion of the amine bands around 3420 cm−1 and the formation of characteristic bands consistent with borazine; 2560 cm−1 (B–H), 1400 cm−1 (B–N stretch).7,11 The intensity of the B–H band which appears as a broad intense band at 2400 cm−1 in the amine-borane adducts is significantly reduced upon borazine formation. We have also collected solid-state 11B and 13C CP-MAS (Fig. S9–S12, ESI) to establish the connectivity and coordination number of boron and to verify the inclusion of intact building units into the framework of BLPs. The 11B signals for both polymers appear as very broad peaks centered around 10.8 ppm for BLP-1(H) and 10.1 ppm for BLP-12(H). These measurements are consistent with reported values for borazine-containing polymers (0 to 40 ppm)12 and are in sharp contrast to tetra-coordinated boron sites found in borane-amine adducts or cycloborazanes (0 to −45 ppm).13 Moreover, the 13C CP-MAS signals which appear as broad peaks in the aromatic range support the incorporation of intact phenyl and tetraphenylmethane cores into the networks of BLP-1(H) and BLP-12(H), respectively.
Given the fact that borazine is isostructural to the boroxine unit found in COFs such as COF-1 and COF-102, we anticipated BLPs to be thermally stable and highly porous. Therefore we subjected BLPs to thermogravimetric analysis and nitrogen porosity measurements. The TGA traces of the as-prepared materials undergo substantial weight loss, presumably due to pore evacuation of solvent molecules and unreacted adducts, upon heating under nitrogen atmosphere and remain stable up to 400 °C (Fig. S15–S16, ESI). This is a typical behavior of porous materials in general and has been reported for porous covalent frameworks.2 Based on TGA studies and the thermal stability of BLPs we activated both materials by degassing at 120 °C and 1 × 10−5 torr for 16 h prior to nitrogen porosity measurements. The Type I nitrogen isotherms (Fig. 1) are consistent with permanent microporous materials that are characterized by a sharp uptake at P/Po = 10−4 to 10−2, while the final rise in the nitrogen uptake is due to condensation in intermolecular cavities created by the packing of BLP particles. The Brunauer-Emmett-Teller (BET) model was applied to each isotherm for P/Po between 0.05 and 0.15 and resulted in surface areas of 1360 and 2244 m2 g−1 for BLP-1(H) and BLP-12(H), respectively. The Langmuir model (P/Po = 0.05–0.30) gave surface area values of 1744 and 2866 m2 g−1. The surface area of BLP-1(H) is considerably higher than related covalent nets: COF-1 (711 m2 g−1),2aCTF-1 (791 m2 g−1),3a and halogenated BLPs (503–1364 m2 g−1),7 while the surface area of BLP-12(H) is among the highest for organic polymers. The pore volumes (Vp) calculated at P/Po = 0.90 were 0.69 cm3 g−1 for BLP-1(H) and 1.08 cm3 g−1 for BLP-12(H). Pore size distributions were estimated using density functional theory (DFT) calculations which revealed narrow pore-size distributions centered at about 12.7 Å.
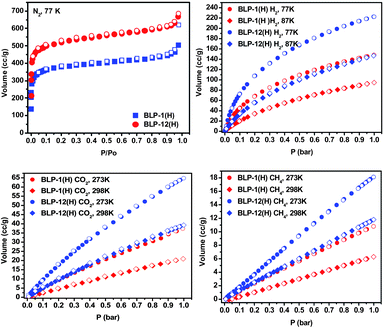 | ||
| Fig. 1 Gas uptake isotherms for BLP-1(H) and BLP-12(H); adsorption (filled) and desorption (empty). | ||
Once the porosity of BLPs had been established, we considered their performance in gas storage. Hydrogen is among the leading candidates for future use in automotive applications because of its abundance, renewability, and clean aspects.14 Physisorbed hydrogen storage is highly attractive because of the rapid uptake and release of hydrogen.15 However, the weak interactions between hydrogen molecules and pore walls necessitate the use of low temperatures and elevated pressure conditions to offset the usually low heat of adsorption. Therefore, developing new materials with enhanced storage properties to meet targets set by the US Department of Energy for 2015 (5.5 wt% hydrogen and 0.040 kg hydrogen/L) remains a considerable challenge.16 We collected hydrogen isotherms at 77 K and 87 K (Fig. 1) and calculated the hydrogen isosteric heats of adsorption (Qst) using the virial method.17 The H2 uptake for BLP-1(H) (1.33 wt%) is lower than that of BLP-12(H) (1.93 wt%) yet both are higher than our previously reported halogenated BLPs (0.68–1.30 wt%).7 Moreover, despite their amorphous nature, the performance of BLPs in hydrogen storage is very comparable with other organic polymers of similar surface areas. For example, under similar conditions the analogous crystalline COF-14a and CTF-13 store 1.28 and 1.55 wt% of H2, respectively, the hydrogen uptake by BLPs is also similar to those of purely organic polymers such as hypercrosslinked polymer networks synthesized by the self-condensation of bischloromethyl monomers (0.89–1.69 wt%),18nitrogen-linked nanoporous networks of aromatic rings (0.01–0.85 wt%),19 and polymers of intrinsic microporosity (PIMs) which recently have been reported to be among the best organic polymers for hydrogen uptake (0.74–1.83 wt%).20 In contrast, activated carbon such as PICACTIF-SC, AX-21, and zeolite-templated show a noticeably higher uptake (1.90, 2.40, and 2.60 wt%, respectively) due to their ultrafine pores.21 The Qst values for hydrogen at low coverage were found to be 6.8 kJ mol−1 (BLP-1(H)) and 6.0 kJ mol−1 (BLP-12(H)) and drop at higher loading to reach 5.5 and 4.8 kJ mol−1, respectively (Fig. S27, S29, ESI). These Qst values are similar to those reported for organic polymers such as POFs (5.8–8.3),4g,h 2D COFs (6.0–7.0 kJ mol−1),4apolyimide networks (5.3–7.0 kJ mol−1),4e,f PPNs (5.5–7.6 kJ mol−1),4i and BILP-1 (7.9 kJ mol−1)5 and are much higher than values reported for 3D COFs: COF-102 (3.9 kJ mol−1), COF-103 (4.4 kJ mol−1), and the carbon-based porous aromatic framework PAF-1 (4.6 kJ mol−1).4c As expected, these Qst values fall below those of the halogenated BLPs (7.1–7.5 kJ mol−1)7 which is most likely due to the higher polarization level of the borazine rings in halogen decorated BLPs.
We have also investigated the performance of BLPs in low pressure CH4 and CO2 storage at 273 K and 298 K (Fig. 1). Interest in storing CH4 stems from its potential in automotive applications due to its abundance and low carbon footprint, whereas CO2 capture or separation from flue gases or coal-fired plants is highly attractive due to environmental and economical reasons.22,23 The maximum CO2 uptake at 273/298 K was 7.4/4.1 wt% (BLP-1(H)) and 12.8/7.4 wt% (BLP-12(H)) with Qst values of 25.3 kJ mol−1 and 25.2 kJ mol−1, respectively. The uptake capacities of BLPs exceed values reported for other organic polymers including diimide based porous organic polymers (POPs) (9.1 and 6.6 wt%),24 and unfunctionalized MOFs which include MIL-53 (9.6 wt%), IRMOF-1 (4.7 wt%), ZIF-100 (4.3 wt%) and MOF-177 (3.5 wt%).22b,c However, CO2 uptakes are lower than those of amine functionalized MOFs or organic polymers and molecules such as HKUST-1 (17.9 wt%), Cu–BTC (17.9 wt%) and BioMOF-11 (26.4 wt%),25 and BILP-1 (18.8 wt%).5 At zero coverage, the Qst values 25.3 kJ mol−1 and 25.2 kJ mol−1 are higher than the values reported for COFs,4aimine-linked organic cages26 or diimide polymers,4e,f and lower than those of CO2-selective MOFs8 or ZTFs10 which generally feature –NH2 or –OH functionalized pores. The maximum uptake for CH4 at 273 K was 13.7 cm3/g for BLP-1(H) and 18.1 cm3/g for BLP-12(H) (Fig. 1) with respective Qst values of 16.7 kJ mol−1 and 17.0 kJ mol−1 at low coverage. A very recent study on the use of COFs illustrated their potential in methane uptake where heat of adsorption depended strongly on pore size and spanned a range of 8 to 25 kJ mol−1, with higher values for narrower pores.23 Both materials reported in this study have relatively similar pore apertures (∼12.7 Å) that resemble those of microporous 2D and 3D COFs.4a The CH4 storage capacity and isosteric heat of adsorption of BLPs are in line with those of COFs and organic polymers under similar conditions.4a,27 Noteworthy are the higher CO2 uptake and binding affinity over CH4 and H2 by BLPs which may arise due to a more favorable interaction between the polarizable CO2 molecules and borazine rings.
In conclusion, we have demonstrated the synthesis of highly porous borazine-linked polymers by thermolysis of arylamine-borane adducts and investigated their performance under ambient pressure and cryogenic conditions. The hydrogen sorption experiments indicate that BLPs have high hydrogen storage capacities at low pressure and display somewhat higher hydrogen isosteric heat of adsorption; however, higher pressure conditions are required to fully explore the potential of BLPs in gas storage applications.
We are grateful to the U. S. Department of Energy, Office of Basic Energy Sciences (DE-SC0002576) for generous support of this project. H.M.E. acknowledges support of the Donors of the American Chemical Society Petroleum Research Fund ACS-PRF (48672-G5).
Notes and references
-
(a) N. B. McKeown and P. M. Budd, Macromolecules, 2010, 43, 5163 CrossRef CAS
; (b) J. X. Jiang and A. I. Cooper, Top. Curr. Chem., 2010, 293, 1 CrossRef CAS
.
-
(a) A. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166 CrossRef
; (b) H. M. El-Kaderi, J. R. Hunt, J. L. Mendoza-Cortés, A. P. Côté, R. E. Taylor, M. O'Keeffe and O. M. Yaghi, Science, 2007, 316, 268 CrossRef CAS
; (c) A. P. Côté, H. M. El-Kaderi, H. Furukawa, J. R. Hunt and O. M. Yaghi, J. Am. Chem. Soc., 2007, 129, 12914 CrossRef
.
-
(a) P. Kuhn, M. Antonietti and A. Thomas, Angew. Chem., Int. Ed., 2008, 47, 3450 CrossRef CAS
; (b) P. Kuhn, A. Forget, D. Su, A. Thomas and M. Antonietti, J. Am. Chem. Soc., 2008, 130, 13333 CrossRef CAS
.
-
(a) H. Furukawa and O. M. Yaghi, J. Am. Chem. Soc., 2009, 25, 8876 Search PubMed
; (b) B. S. Ghanem, M. Hashem, K. D. M. Harris, K. J. Msayib, M. Xu, P. M. Budd, N. Chaukura, D. Book, S. Tedds, A. Walton and N. B. McKeown, Macromolecules, 2010, 43, 5287 CrossRef CAS
; (c) T. Ben, H. Ren, S. Ma, D. Cao, J. Lan, X. Jing, W. Wang, J. Xu, F. Deng, J. M. Simmons, S. Qiu and G. Zhu, Angew. Chem., Int. Ed., 2009, 48, 9457 CrossRef CAS
; (d) R. M. Kassab, K. T. Jackson, O. M. El-Kadri and H. M. El-Kaderi, Res. Chem. Intermed., 2011, 37, 747 CrossRef CAS
; (e) Z. Wang, B. Zhang, H. Yu, L. Sun, C. Jiaob and E. Liua, Chem. Commun., 2010, 46, 7730 RSC
; (f) O. K. Farha, A. M. Spokoyny, B. G. Hauser, Y.-S. Bae, S. E. Brown, R. Q. Snurr, C. A. Mirkin and J. T. Hupp, Chem. Mater., 2009, 21, 3033 CrossRef CAS
; (g) P. Pandey, A. P. Katsoulidis, I. Eryazici, Y. Wu, M. G. Kanatzidis and S. T. Nguyen, Chem. Mater., 2010, 22, 4974 CrossRef CAS
; (h) A. P. Katsoulidis and M. G. Kanatzidis, Chem. Mater., 2011, 23, 1818 CrossRef CAS
; (i) W. Lu, D. Yuan, D. Zhao, C. I. Schilling, O. Plietzsch, T. Muller, S. Bräse, J. Guenther, J. Blümel, R. Krishna, Z. Li and H.-C. Zhou, Chem. Mater., 2010, 22, 5964 CrossRef CAS
.
- M. G. Rabbani and H. M. El-Kaderi, Chem. Mater., 2011, 23, 1650 CrossRef CAS
.
- J. L. Belof, A. C. Stern, M. Eddaoudi and B. Space, J. Am. Chem. Soc., 2007, 129, 15202 CrossRef CAS
.
- T. E. Reich, K. T. Jackson, S. Li, P. Jena and H. M. El-Kaderi, J. Mater. Chem., 2011, 21, 10629 RSC
.
-
(a) V. Salles, S. Bernard, J. Li, A. Brioude, S. Chehaidi, S. Foucaud and P. Miele, Chem. Mater., 2009, 21, 2920 CrossRef CAS
; (b) S. Duperrier, S. Bernard, A. Calin, C. Sigala, R. Chiriac, P. Miele and C. Balan, Macromolecules, 2007, 40, 1028 CrossRef CAS
.
- I. H. T. Sham, C.-C. Kwok, C.-M. Che and N. Zhu, Chem. Commun., 2005, 3547 RSC
.
-
(a) A. Wakamiya, T. Ide and S. Yamaguchi, J. Am. Chem. Soc., 2005, 127, 14859 CrossRef CAS
; (b) S. Yamaguchi and A. Wakamiya, Pure Appl. Chem., 2006, 78, 1413 CrossRef CAS
.
-
(a) A. Kaldor and R. F. Porter, Inorg. Chem., 1971, 10, 775 CrossRef CAS
; (b) J. S. Li, C. R. Zhang, B. Li, F. Cao and S. Q. Wang, Inorg. Chim. Acta, 2011, 366, 173 CrossRef CAS
; (c) K. T. Moon, D. S. Min and D. P. Kim, J. Ind. Eng. Chem., 1997, 3, 288 CAS
.
-
(a) C. Gervais and F. Babonneau, J. Organomet. Chem., 2002, 657, 75 CrossRef CAS
; (b) C. Gervais, E. Framery, C. Duriez, J. Maquet, M. Vaultier and F. Babonneau, J. Eur. Ceram. Soc., 2005, 25, 129 CrossRef CAS
.
-
(a) C. Gervais, F. Babonneau, J. Maquet, C. Bonhomme, D. Massiot, E. Framery and M. Vaultier, Magn. Reson. Chem., 1998, 36, 407 CrossRef CAS
; (b) J. Li, C. Zhang, B. Li, F. Cao and S. Wang, Eur. J. Inorg. Chem., 2010, 1763 CrossRef CAS
; (c) X. Chen, X. Bao, J.-C. Zhao and S. G. Shore, J. Am. Chem. Soc., 2011, 133, 14172 CrossRef CAS
.
- L. Schlapbach and A. Zuttel, Nature, 2001, 414, 353 CrossRef CAS
.
-
(a) L. J. Murray, M. Dinca and J. R. Long, Chem. Soc. Rev., 2009, 38, 1294 RSC
; (b) H.-C. Zhou, Chem. Commun., 2010, 46, 44 RSC
; (c) J. Germain, J. M. J. Fréchet and F. Svec, Small, 2009, 5, 1098 CrossRef CAS
.
- U.S. Department of Energy, Energy Efficiency and Renewable Energy. http://www1.eere.energy.gov/hydrogenandfuelcells/storage/current_technology.html (accessed Mar 25, 2011).
- J. L. C. Rowsell and O. M. Yaghi, J. Am. Chem. Soc., 2006, 128, 1304 CrossRef CAS
.
- C. D. Wood, B. Tan, A. Trewin, H. J. Niu, D. Bradshaw, M. J. Rosseinsky, Y. Z. Khimyak, N. L. Campbell, R. Kirk, E. Stockel and A. I. Cooper, Chem. Mater., 2007, 19, 2034 CrossRef CAS
.
- J. Germain, F. Svec and J. M. J. Fréchet, Chem. Mater., 2008, 20, 7069 CrossRef CAS
.
- B. S. Ghanem, M. Hashem, K. D. M. Harris, K. J. Msayib, M. C. Xu, P. M. Budd, N. Chaukura, D. Book, S. Tedds, A. Walton and N. B. McKeown, Macromolecules, 2010, 43, 5287 CrossRef CAS
.
-
(a) N. Texier-Mandoki, J. Dentzer, T. Piquero, S. Saadallah, P. David and C. Vix-Guterl, Carbon, 2004, 42, 2744 CrossRef CAS
; (b) Z. X. Yang, Y. D. Xia and R. Mokaya, J. Am. Chem. Soc., 2007, 129, 1673 CrossRef CAS
.
-
(a) D. M. D'Alessandro, B. Smit and J. R. Long, Angew. Chem., Int. Ed., 2010, 49, 2 CrossRef
; (b) S. Q. Ma and H. C. Zhou, Chem. Commun., 2010, 46, 44 RSC
; (c) S. Keskin, T. M. van Heest and D. S. Sholl, ChemSusChem, 2010, 3, 879 CrossRef CAS
.
- J. L. J. Mendoza-Cortés, S. S. Han, H. Furukawa, O. M. Yaghi and W. A. Goddard, J. Phys. Chem. A, 2010, 114, 10824 CrossRef
.
- O. K. Farha, Y.-S. Bae, B. G. Hauser, A. M. Spokoyny, R. Q. Snurr, C. A. Mirkin and J. T. Hupp, Chem. Commun., 2010, 46, 1056 RSC
.
- J. An, S. J. Geib and N. L. Rosi, J. Am. Chem. Soc., 2010, 132, 38 CrossRef CAS
.
- Y. Jin, B. A. Voss, A. Jin, H. Long, R. D. Noble and W. Zhang, J. Am. Chem. Soc., 2011, 133, 6650 CrossRef CAS
.
- M. G. Schwab, A. Lennert, J. Pahnke, G. Jonschker, M. Koch, I. Senkovska, M. Rehahn and S. Kaskel, J. Mater. Chem., 2011, 21, 2131 RSC
.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental procedures, characterization methods, and gas sorption studies. See DOI: 10.1039/c1py00374g/ |
| This journal is © The Royal Society of Chemistry 2011 |
